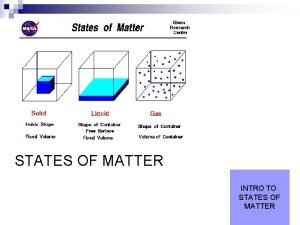
Chemistry Intro Notes Chemistry Study of matter and

- Slides: 10

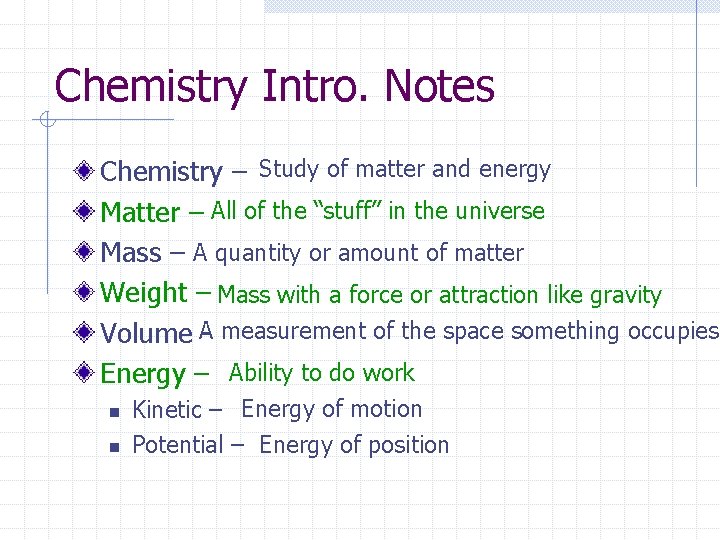
Chemistry Intro. Notes Chemistry – Study of matter and energy Matter – All of the “stuff” in the universe Mass – A quantity or amount of matter Weight – Mass with a force or attraction like gravity Volume A- measurement of the space something occupies Energy – Ability to do work n n Kinetic – Energy of motion Potential – Energy of position

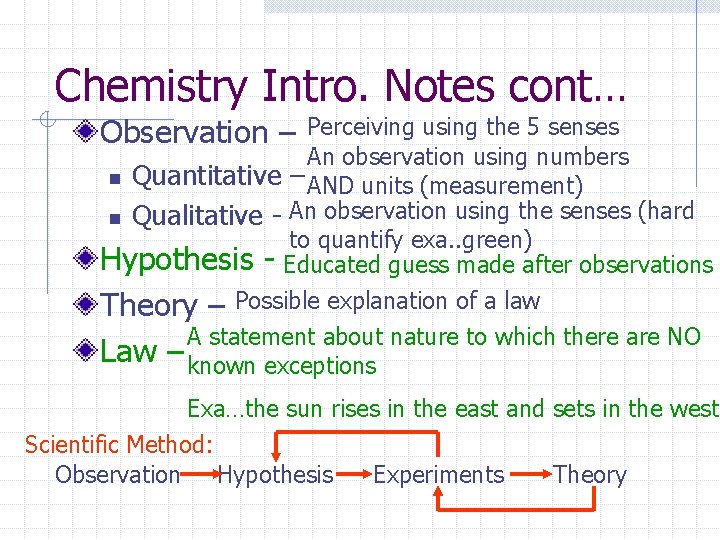
Chemistry Intro. Notes cont… Observation – Perceiving using the 5 senses An observation using numbers n Quantitative – AND units (measurement) n Qualitative - An observation using the senses (hard to quantify exa. . green) Hypothesis - Educated guess made after observations Theory – Possible explanation of a law A statement about nature to which there are NO Law – known exceptions Exa…the sun rises in the east and sets in the west Scientific Method: Observation Hypothesis Experiments Theory

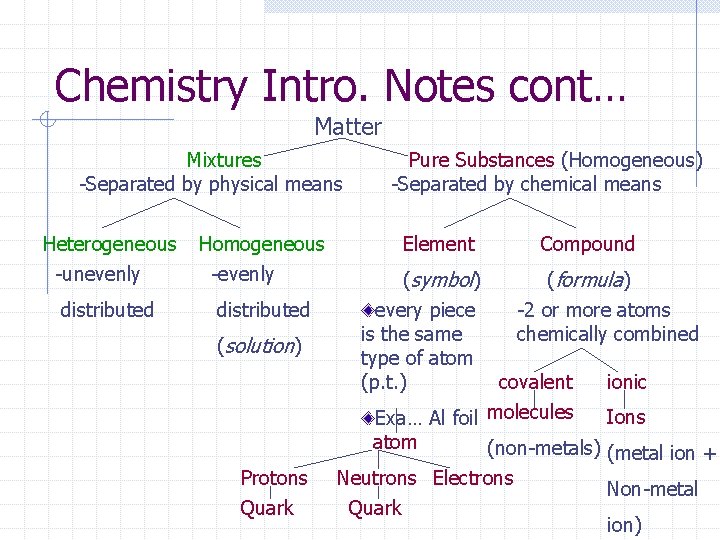
Chemistry Intro. Notes cont… Matter Mixtures -Separated by physical means Heterogeneous -unevenly distributed Homogeneous -evenly distributed (solution) Protons Quark Pure Substances (Homogeneous) -Separated by chemical means Element Compound (symbol) (formula) every piece -2 or more atoms is the same chemically combined type of atom covalent ionic (p. t. ) Ions Exa… Al foil molecules atom (non-metals) (metal ion + Neutrons Electrons Non-metal Quark ion)

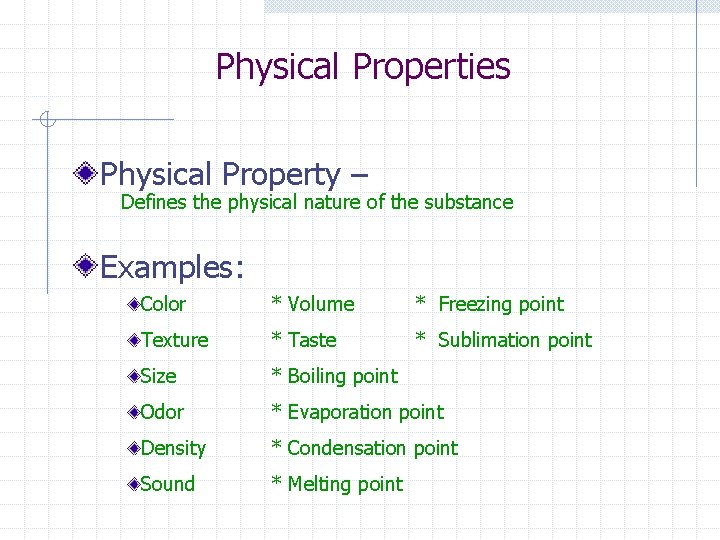
Physical Properties Physical Property – Defines the physical nature of the substance Examples: Color * Volume * Freezing point Texture * Taste * Sublimation point Size * Boiling point Odor * Evaporation point Density * Condensation point Sound * Melting point

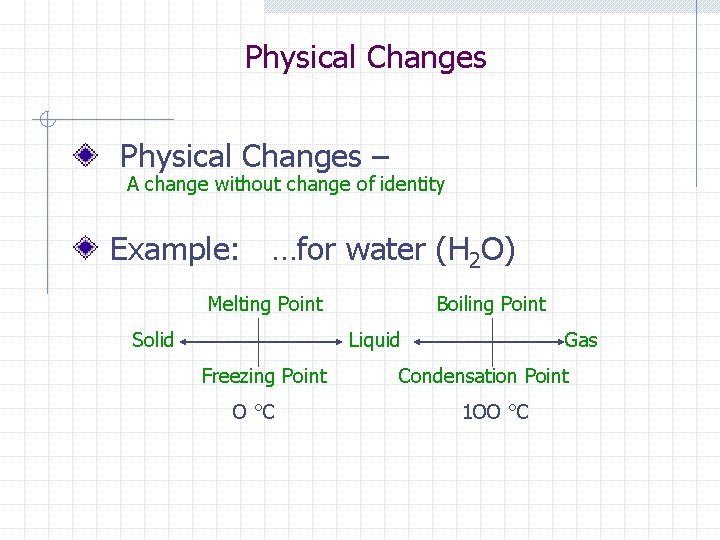
Physical Changes – A change without change of identity Example: …for water (H 2 O) Melting Point Solid Boiling Point Liquid Freezing Point O °C Gas Condensation Point 1 OO °C

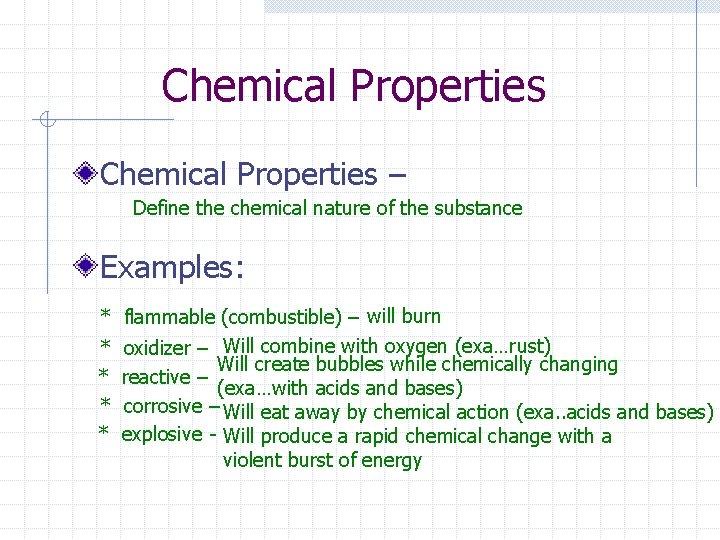
Chemical Properties – Define the chemical nature of the substance Examples: * flammable (combustible) – will burn * oxidizer – Will combine with oxygen (exa…rust) Will create bubbles while chemically changing * reactive – (exa…with acids and bases) * corrosive – Will eat away by chemical action (exa. . acids and bases) * explosive - Will produce a rapid chemical change with a violent burst of energy

Chemical Changes Change with change of identity Chemical change – Evidence of a Chemical change: 1. Light 2. Odor Change 3. Color change (careful!! This can be physical!) 4. Heat Change 5. Reaction, gas given off (NOT dissolving) 6. Precipitate ie “chunky stuff” (filtration) 7. Taste Change 8. Appearance change

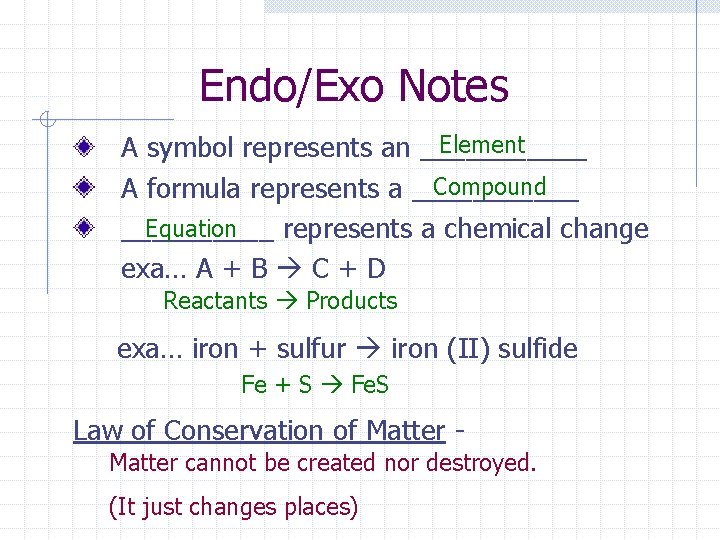
Endo/Exo Notes Element A symbol represents an ______ Compound A formula represents a ______ Equation _____ represents a chemical change exa… A + B C + D Reactants Products exa… iron + sulfur iron (II) sulfide Fe + S Fe. S Law of Conservation of Matter cannot be created nor destroyed. (It just changes places)

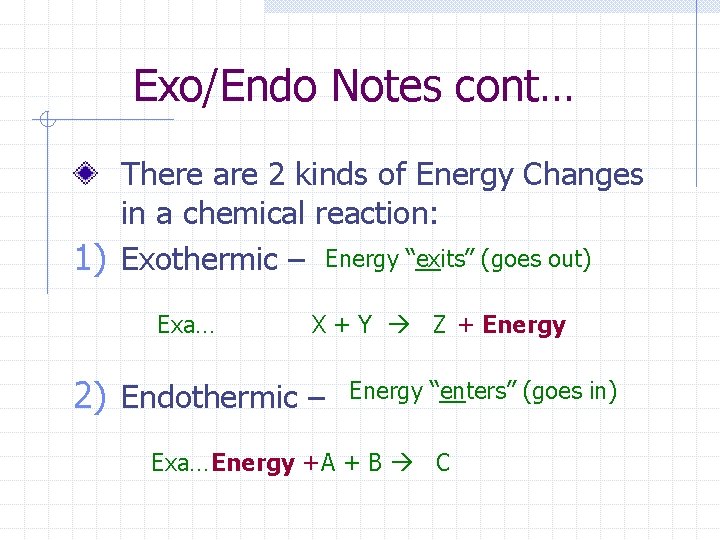
Exo/Endo Notes cont… There are 2 kinds of Energy Changes in a chemical reaction: 1) Exothermic – Energy “exits” (goes out) Exa… X + Y Z + Energy 2) Endothermic – Energy “enters” (goes in) Exa… Energy + A + B C

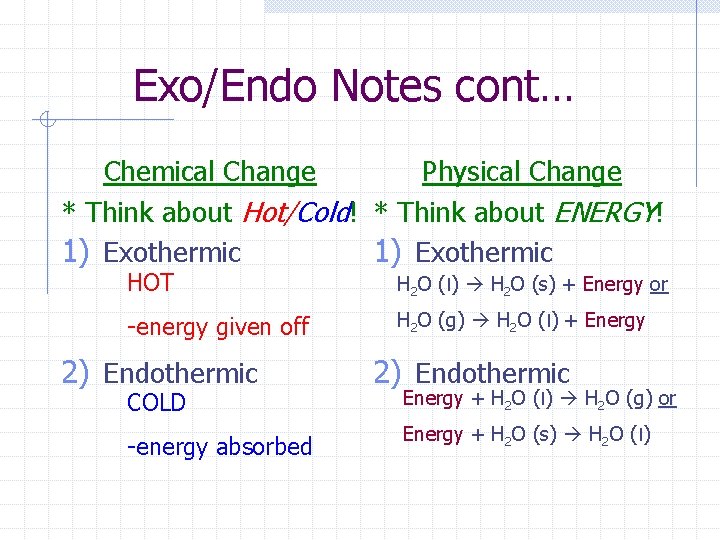
Exo/Endo Notes cont… Chemical Change Physical Change * Think about Hot/Cold! * Think about ENERGY! 1) Exothermic HOT H 2 O (l) H 2 O (s) + Energy or -energy given off H 2 O (g) H 2 O (l) + Energy 2) Endothermic COLD -energy absorbed 2) Endothermic Energy + H 2 O (l) H 2 O (g) or Energy + H 2 O (s) H 2 O (l)
 Intro to matter
Intro to matter Function of grey matter and white matter
Function of grey matter and white matter Brain falx
Brain falx Gray matter and white matter
Gray matter and white matter Telencephalon
Telencephalon Importance of organic compounds
Importance of organic compounds Intro to business chapter 7 study guide
Intro to business chapter 7 study guide Classification of matter section 1 composition of matter
Classification of matter section 1 composition of matter Classification of matter section 1 composition of matter
Classification of matter section 1 composition of matter Chapter 2 section 1 classifying matter answer key
Chapter 2 section 1 classifying matter answer key Section 1 composition of matter chapter 15 answer key
Section 1 composition of matter chapter 15 answer key