Chemical Weathering and Soils Fresh rocks and minerals
















- Slides: 35

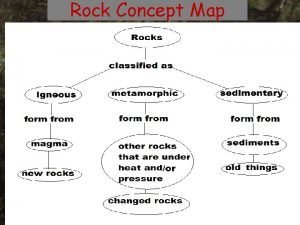
Chemical Weathering and Soils Fresh rocks and minerals that once occupied the outermost position on the Earth’s surface reached their present condition of decay through a complex of interacting physical, chemical, and biological processes, collectively called weathering. 1. new minerals created by the weathering processes, 2. minerals that resisted destruction, and 3. organic debris added to the weathering zone.

Chemical Weathering and Soils Functions of weathering • reduces rock strength and increases permeability, rendering it more susceptible to mass wasting and erosion; reduces strength (cohesion and friction) and increases permeability of rock and therefore decreases resistance to fluid and gravitational stresses; precursor to erosion • produces minor landforms, produces landforms in soluble rock (especially limestone) and otherwise creates microrelief (e. g. weathering pits) • releases minerals in solution (e. g. iron oxides, silica, carbonates) which become concentrated to form hard coatings on rocks and hard resistant layers in soil (duricrusts) that inhibit seepage and resist erosion • first step in soil formation; ultimately produces an unconsolidated mass of 1) minerals that resisted alteration (e. g. feldspar), 2) new minerals (e. g. bauxite), 3) organic debris

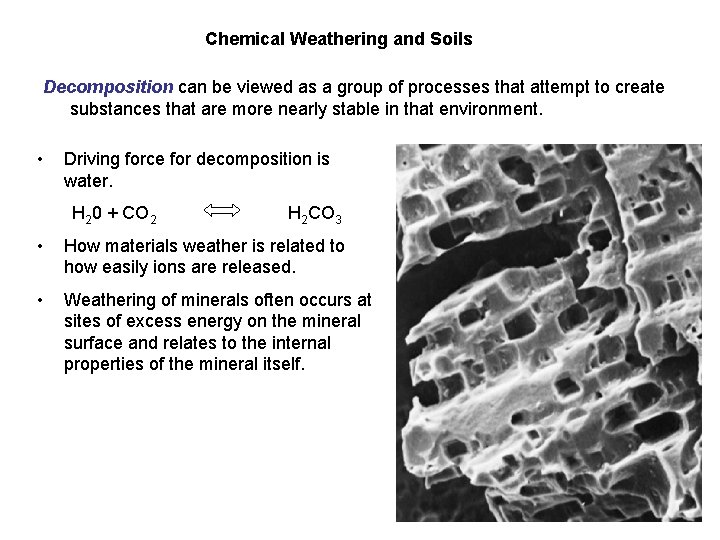
Chemical Weathering and Soils Decomposition can be viewed as a group of processes that attempt to create substances that are more nearly stable in that environment. • Driving force for decomposition is water. H 20 + CO 2 H 2 CO 3 • How materials weather is related to how easily ions are released. • Weathering of minerals often occurs at sites of excess energy on the mineral surface and relates to the internal properties of the mineral itself.

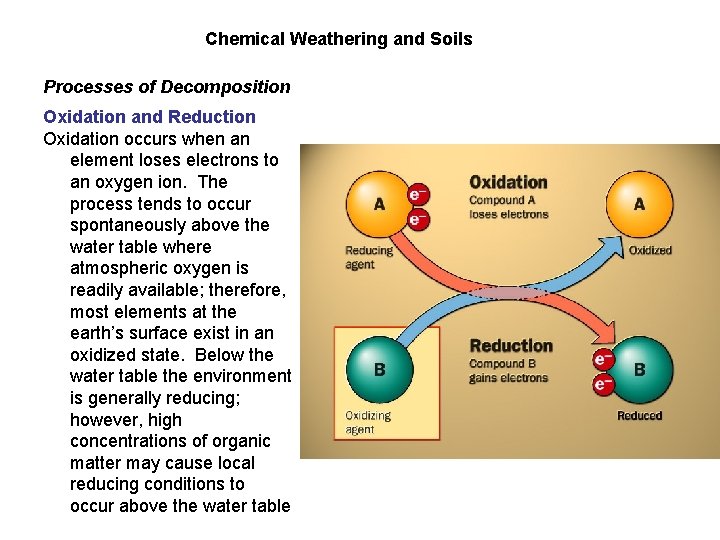
Chemical Weathering and Soils Processes of Decomposition Oxidation and Reduction Oxidation occurs when an element loses electrons to an oxygen ion. The process tends to occur spontaneously above the water table where atmospheric oxygen is readily available; therefore, most elements at the earth’s surface exist in an oxidized state. Below the water table the environment is generally reducing; however, high concentrations of organic matter may cause local reducing conditions to occur above the water table

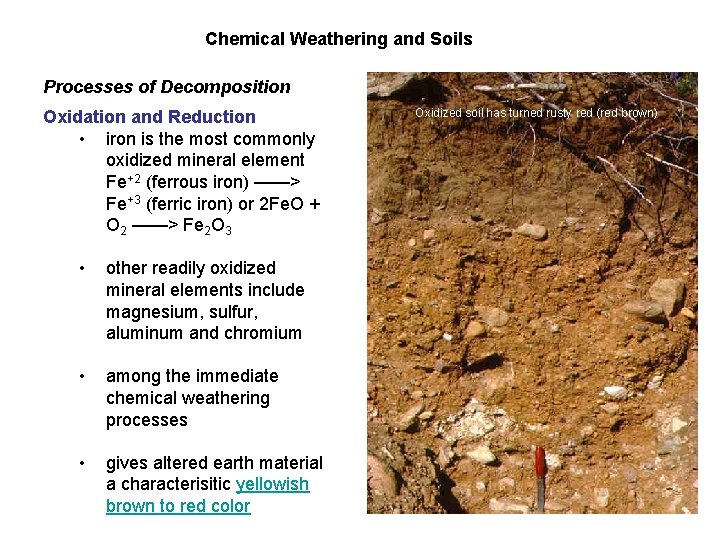
Chemical Weathering and Soils Processes of Decomposition Oxidation and Reduction • iron is the most commonly oxidized mineral element Fe+2 (ferrous iron) ——> Fe+3 (ferric iron) or 2 Fe. O + O 2 ——> Fe 2 O 3 • other readily oxidized mineral elements include magnesium, sulfur, aluminum and chromium • among the immediate chemical weathering processes • gives altered earth material a characterisitic yellowish brown to red color Oxidized soil has turned rusty red (red brown)

Chemical Weathering and Soils Processes of Decomposition Solution Is critical in chemical weathering because when atoms are dissolved from a mineral, the structure becomes unstable. However, the precise way in which a mineral collapses varies with its crystalline structure and the mobility of its constituent ions. • dissolution of calcium carbonate in acidic soil and groundwater Ca. CO 3 + H 2 CO 3 ——> Ca+2 + 2 HCO 3 - • similar reaction as hydrolosis but the dissolution is congruent, that is, the products are ionic, there is no residue • bicarbonate represents the largest constituent of the dissolved load of most rivers • carbonation of limestone results in karst topography • the insoluble minerals form soil

Chemical Weathering and Soils Processes of Decomposition Hydrolysis Chemically, hydrolysis involves a reaction between a salt and water to produce an acid and a base; it is probably the most important mechanism in breaking apart structures of the silicate minerals. During the process, metallic cations are separated from the mineral structure and replaced by H+, which is held in the original mineral complex. Most of the replaced cations are soluble in natural waters. • decomposition of minerals in water as hydrogen ions replace cations in minerals • pure water is a poor H+ donor, however CO 2 dissolves in water to produce carbonic acid: CO 2 + H 2 O H 2 CO 3 (carbonic acid) H++ HCO 3 - (bicarbonate)

Chemical Weathering and Soils Processes of Decomposition Hydrolysis • soil air is greatly enriched in CO 2 by decay of humus • up to 30% of soil air is CO 2 as compared to 0. 03% of the atmosphere • biogenic CO 2 is the major source of carbonated groundwater • solubility of CO 2 increases as water temperature decreases (warm beer is flat) • hydrolysis is the most important process in the weathering of silicate minerals • the other weathering products (silicic acid and ions) are in solution, so the residue is clay • the soil water solution becomes more basic as H+ is consumed

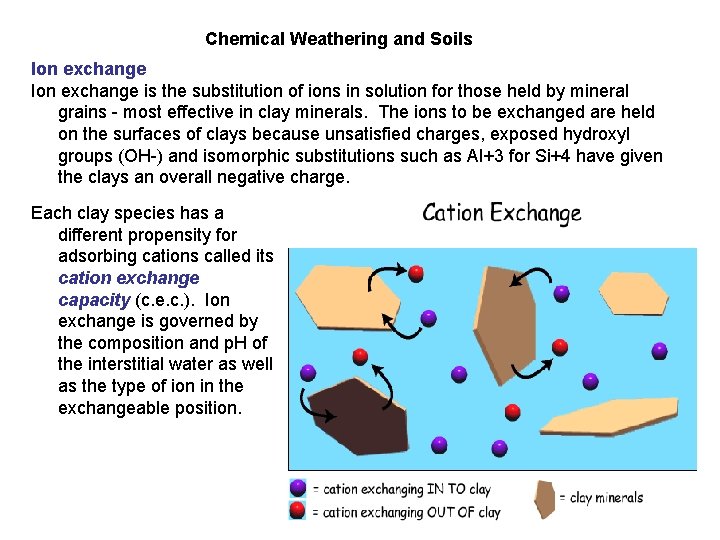
Chemical Weathering and Soils Ion exchange is the substitution of ions in solution for those held by mineral grains - most effective in clay minerals. The ions to be exchanged are held on the surfaces of clays because unsatisfied charges, exposed hydroxyl groups (OH-) and isomorphic substitutions such as Al+3 for Si+4 have given the clays an overall negative charge. Each clay species has a different propensity for adsorbing cations called its cation exchange capacity (c. e. c. ). Ion exchange is governed by the composition and p. H of the interstitial water as well as the type of ion in the exchangeable position.

Chemical Weathering and Soils Mobility of decomposed materials The extent to which chemical weathering will alter the parent mineralogy depends largely on the relative mobility of the constituent ions. Some ions are easily removed (high mobility) from the weathering system under normal groundwater conditions, while others are relatively difficult to remove (high immobility). 1. Leaching - the significance of leaching is several fold: • it removes in solution the constituents that have been separated from minerals by hydrolysis and ion exchange and, by providing new hydrogen, allows these processes continuously to alter the original material toward an ultimate degraded condition, • it directly affects the p. H of fluids surrounding minerals and thereby helps determine which elements will remain in solution, and • it provides the mechanism by which dissolved ions and clays are transferred from higher levels to lower levels in the weathering zone.

Chemical Weathering and Soils Mobility of decomposed materials 2. Fixation and retardation Some cations, especially potassium, have a tendency to be retained or fixed in the weathered zone, so that their mobility is considerably lower than might be expected. The precise mechanism involved is poorly understood, but is probably related to the fact that potassium silicates are more resistant to chemical weathering than are other minerals with similar lattices. 3. Chelation Defined as the equilibrium reaction between a metal ion and a complexing agent, characterized by the formation of more than one bond between the metal and a molecule of the complexing agent and resulting in the formation of a ring structure incorporating the metal ion. This means that metallic ions that are extremely immobile under normal conditions can be mobilized by reacting with complexing agents and be vertically transported as part of the compound. Most complexing agents involved in chelation are organic compounds nurtured in soils by alteration of humus into a plant acid called fulvic acid.

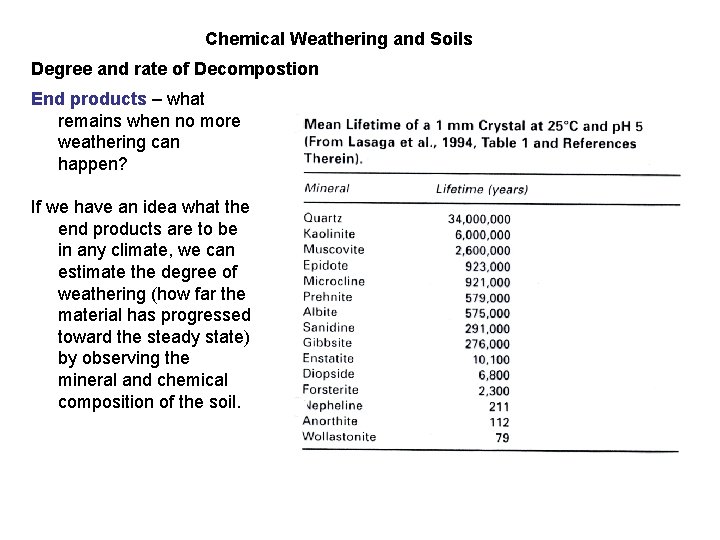
Chemical Weathering and Soils Degree and rate of Decompostion End products – what remains when no more weathering can happen? If we have an idea what the end products are to be in any climate, we can estimate the degree of weathering (how far the material has progressed toward the steady state) by observing the mineral and chemical composition of the soil.

Chemical Weathering and Soils Degree and rate of Decompostion Mineral stability “What minerals will be most rapidly destroyed in a thoroughly leached open system? ” “Which types will remain if a steady state is attained? ” Basically the guide to mineral stability is the inverse of Bowen’s reaction Series (with some exceptions). The minerals that are most out of touch with their formation conditions (hi temps and pressures for example) are the least stable at these surface conditions and are most susceptible to weathering.

Chemical Weathering and Soils Degree and rate of Decompostion Secondary minerals These secondary minerals, born within the weathered zone, are distinctly more stable than their primary ancestors because they reach equilibrium in the temperature-pressure environment of the soil rather than in the magmatic or metamorphic conditions that created the original crystals. Estimates based on chemical analyses The rate of chemical weathering is also commonly estimated by chemical analyses of water that has filtered through the rock and soil system. One assumes, however, that the chemistry of the water is solely a function of the chemistry of the parent material. Not realistic in most instances.

Chemical Weathering and Soils Weathering processes that continue over an extended period of time result in an unconsolidated mass of soil that is measureably different from the original rock/parent material in its physical and chemical properties. Relationship between bedrock and soil profile Soil Profile Bedrock

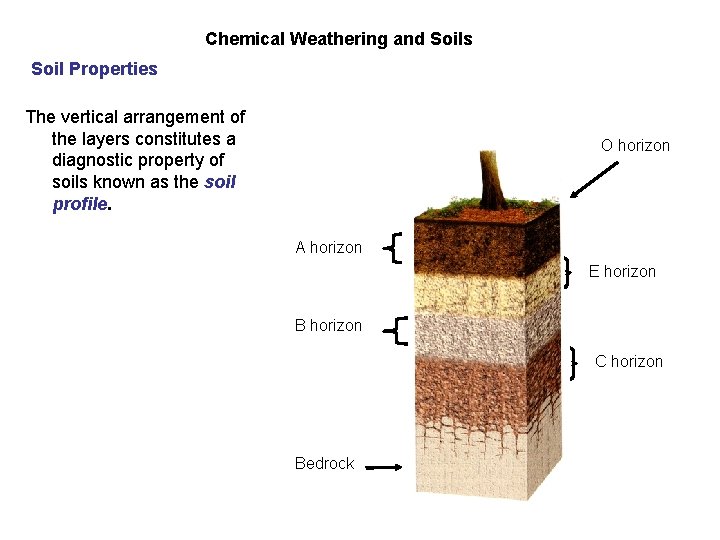
Chemical Weathering and Soils Soil Properties Soil Profile The vertical arrangement of the layers constitutes a diagnostic property of soils known as the soil profile. O horizon A horizon E horizon B horizon C horizon Bedrock

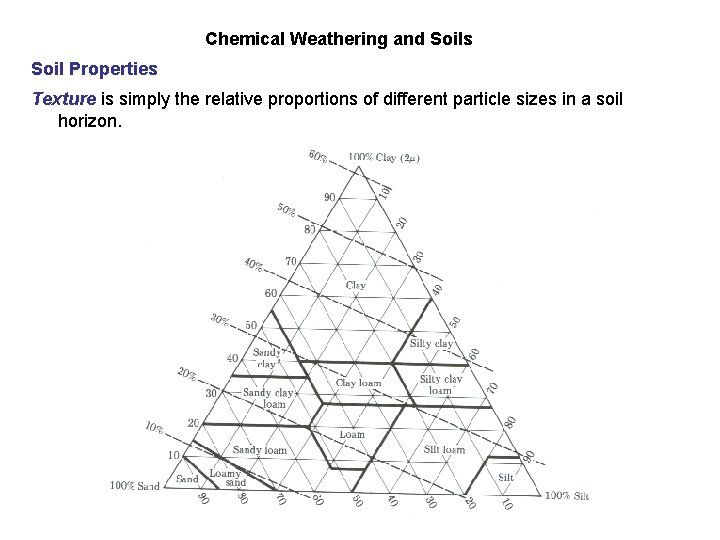
Chemical Weathering and Soils Soil Properties Texture is simply the relative proportions of different particle sizes in a soil horizon.

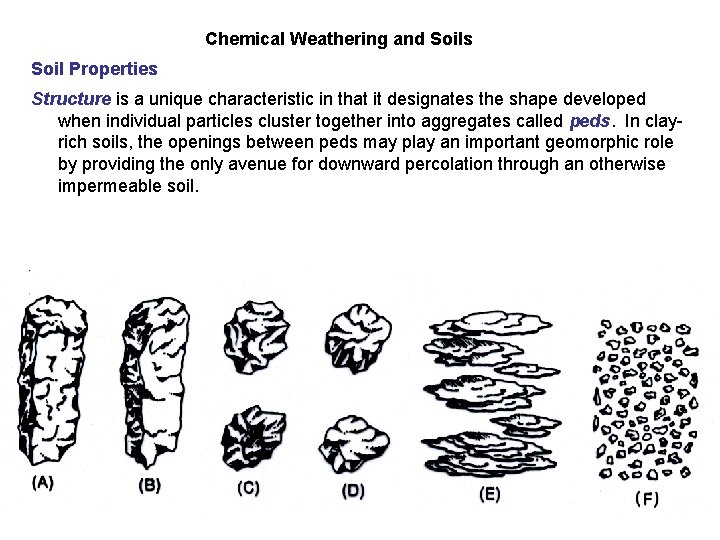
Chemical Weathering and Soils Soil Properties Structure is a unique characteristic in that it designates the shape developed when individual particles cluster together into aggregates called peds. In clayrich soils, the openings between peds may play an important geomorphic role by providing the only avenue for downward percolation through an otherwise impermeable soil.

Chemical Weathering and Soils Soil Properties Organic matter in soils consists mainly of dead leaves, branches, and the like called litter, and the amorphous residue called humus, that develops when litter is decomposed.

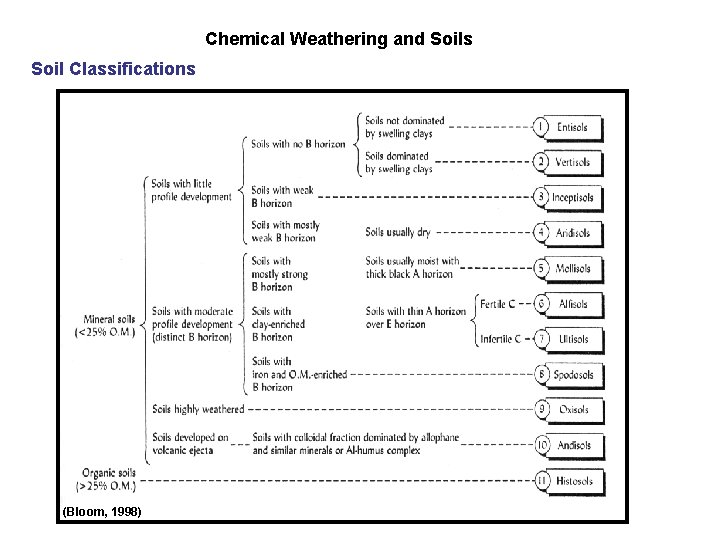
Chemical Weathering and Soils Soil Classifications The National Resources Conservation Service (Dept. of Agriculture) has developed a classification system that is nongenetic and requires no climatic interpretation. Soils are classified into ten major orders distinguished by the major horizons in their profiles. Entisols Vertisols Inceptisols Aridisols Mollisols - Spodosol Alfisol Ultisol Oxisol Histosol - recent inverts inception arid mollify (to lessen in intensity, to soften podzol, odd pedalfer ultimate oxide histology (tissue)

Chemical Weathering and Soils Soil Classifications (Bloom, 1998)

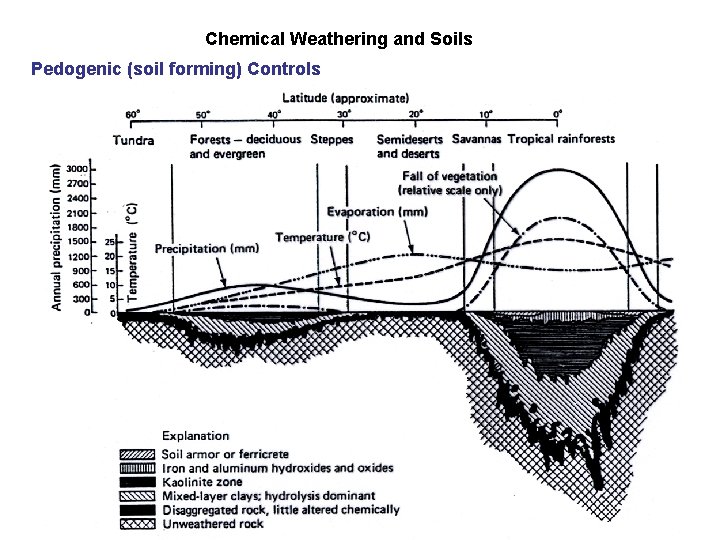
Chemical Weathering and Soils Pedogenic (soil forming) Controls Soil = ∫ (climate, biota, time, parent material and topography) Of these, time is thought to be the most important: • • • horizon development, clay mineralogy, total soil morphology, iron oxide composition, Ca. CO 3 concentration.

Chemical Weathering and Soils Pedogenic (soil forming) Controls Recognize that the value of each property changes at a different rate and that given enough time, each will reach a condition where the property no longer changes or its rate of change is negligible. Climate: The regional climate; because precipitation and temperature are not interdependent, they can be treated as separate functions. Organisms of biotic factor: Because vegetation is most important, this factor is the summation of plant disseminules reaching the soil site or the potential vegetation; it is approximated by a list of species growing in the surrounding region that could gain access to the site under appropriate conditions.

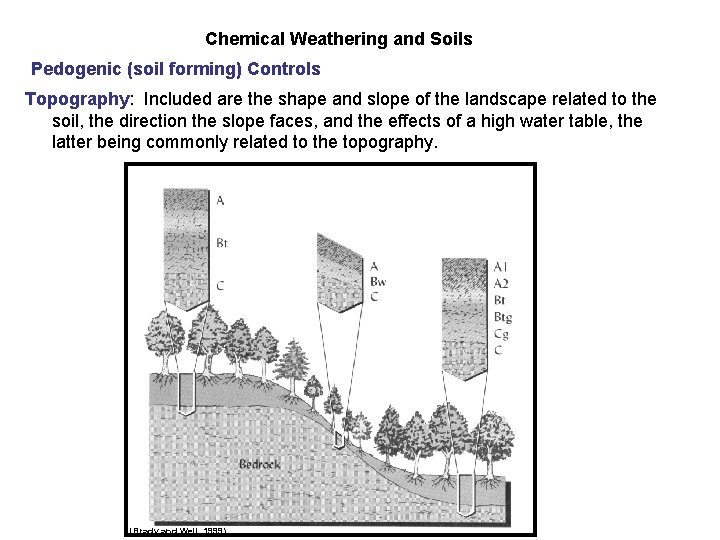
Chemical Weathering and Soils Pedogenic (soil forming) Controls Topography: Included are the shape and slope of the landscape related to the soil, the direction the slope faces, and the effects of a high water table, the latter being commonly related to the topography. (Brady and Weil, 1999)

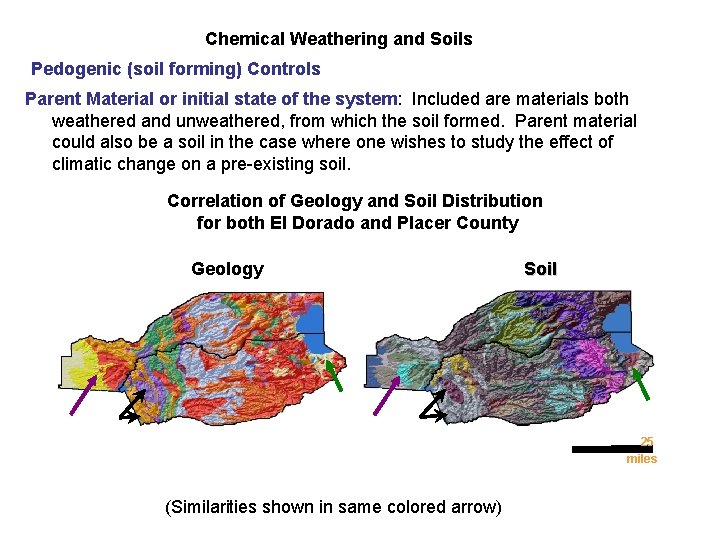
Chemical Weathering and Soils Pedogenic (soil forming) Controls Parent Material or initial state of the system: Included are materials both weathered and unweathered, from which the soil formed. Parent material could also be a soil in the case where one wishes to study the effect of climatic change on a pre-existing soil. Correlation of Geology and Soil Distribution for both El Dorado and Placer County Geology Soil 25 miles (Similarities shown in same colored arrow)

Chemical Weathering and Soils Pedogenic (soil forming) Controls Time: Elapsed time since deposition of material, the exposure of the material at the surface or formation of the slope to which the soil relates; if a study is being made of the effect of climatic change on a preexisting soils, the time since the change.

Chemical Weathering and Soils Pedogenic (soil forming) Controls

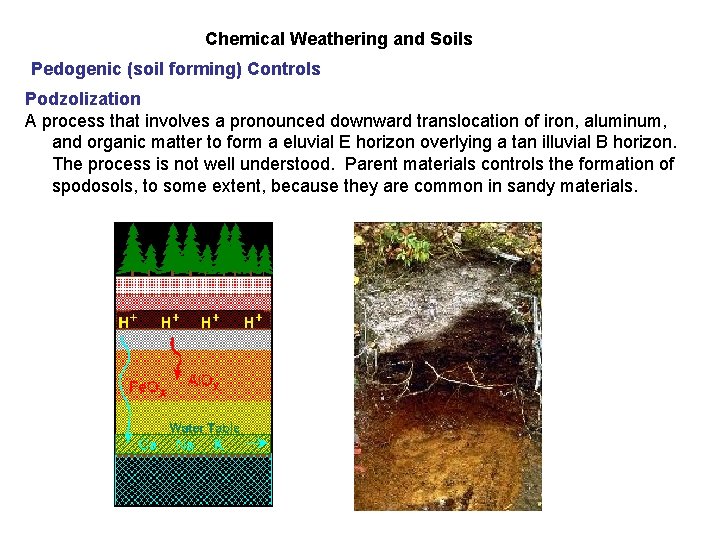
Chemical Weathering and Soils Pedogenic (soil forming) Controls Podzolization A process that involves a pronounced downward translocation of iron, aluminum, and organic matter to form a eluvial E horizon overlying a tan illuvial B horizon. The process is not well understood. Parent materials controls the formation of spodosols, to some extent, because they are common in sandy materials.

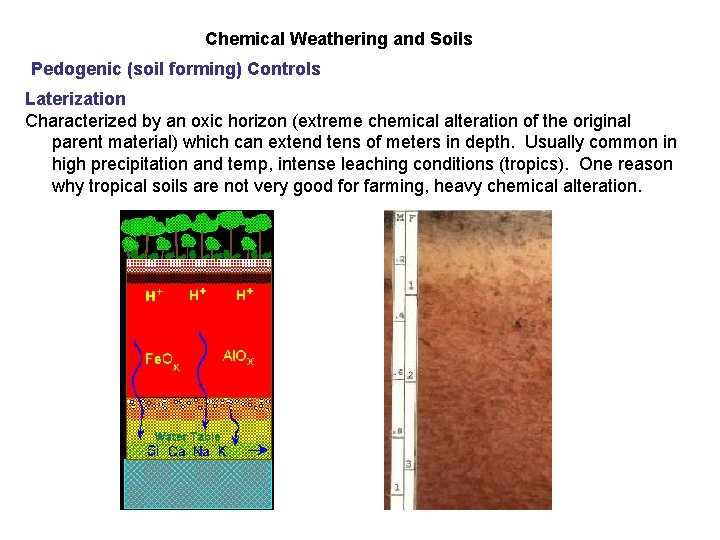
Chemical Weathering and Soils Pedogenic (soil forming) Controls Laterization Characterized by an oxic horizon (extreme chemical alteration of the original parent material) which can extend tens of meters in depth. Usually common in high precipitation and temp, intense leaching conditions (tropics). One reason why tropical soils are not very good for farming, heavy chemical alteration.

Chemical Weathering and Soils Pedogenic (soil forming) Controls Calcification A general buildup of calcium carbonate in the soil profile. Occurs in sub-humid to arid environments where the precipitation is inadequate to drive soil water to the water table so the Ca 2+ ions re-precipitate as a Ca. CO 3 rich B horizon.

Chemical Weathering and Soils Pedogenic (soil forming) Controls Salinization occurs in warm and dry locations where soluble salts precipitate from water and accumulate in the soil. Saline soils are common in desert and steppe climates. Salt may also accumulate in soils from sea spray.

Chemical Weathering and Soils Geomorphic significance of soils Because soils properties are altered by time and climate change, soil formation has even greater importance in deciphering the sequence of events in Quaternary history. In fact, one of the greatest uses of weathering and soils is to establish relative ages of glacial deposits and, by inference, the sequence of glaciations. They are used primarily • in the subdivision of a local succession of deposits,

Chemical Weathering and Soils Geomorphic significance of soils They are used primarily • to provide data on the lengths of time that separate periods of deposition, • to facilitate short- and long-term correlation, and, of recent discovery, • to date Quaternary faulting episodes. • Other more recent applications include archeological studies where soils have been used to indicate the ages of certain layers, past moisture conditions, previous vegetation conditions.

Chemical Weathering and Soils Geomorphic significance of soils The use of soils in Quaternary geomorphology is a dual-edged sword. In areas that have experienced several climatic variations, the soil profile may reflect any or all of these variations. These polygenetic soils (complex soils) complicate the record because thickness and maturity of soils are reliable indices of age only when the conditions developing the soils have been maintained continuously.

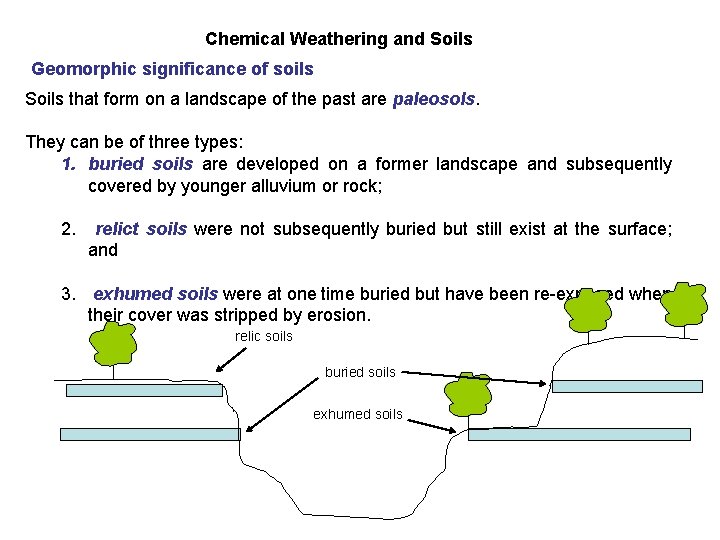
Chemical Weathering and Soils Geomorphic significance of soils Soils that form on a landscape of the past are paleosols. They can be of three types: 1. buried soils are developed on a former landscape and subsequently covered by younger alluvium or rock; 2. relict soils were not subsequently buried but still exist at the surface; and 3. exhumed soils were at one time buried but have been re-exposed when their cover was stripped by erosion. relic soils buried soils exhumed soils
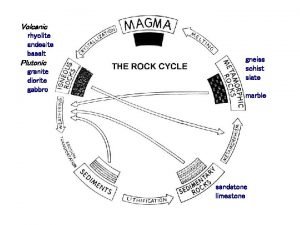
 Rock cycle song (sedimentary igneous metamorphic)
Rock cycle song (sedimentary igneous metamorphic) Igneous rock to metamorphic rock
Igneous rock to metamorphic rock Physical weathering and chemical weathering venn diagram
Physical weathering and chemical weathering venn diagram Three types of weathering
Three types of weathering Chemical weathering examples
Chemical weathering examples Rock cycle song
Rock cycle song Solid rock
Solid rock Difference between rocks and minerals
Difference between rocks and minerals Concept map of classification of rocks
Concept map of classification of rocks Suzanna socked me sunday poem
Suzanna socked me sunday poem Difference between rock and stone
Difference between rock and stone Rocks and minerals
Rocks and minerals Quartzite rock cycle
Quartzite rock cycle Rocks are aggregates of minerals
Rocks are aggregates of minerals What types of weathering are there
What types of weathering are there Intrusive vs extrusive igneous rocks
Intrusive vs extrusive igneous rocks Volcanic rocks and plutonic rocks
Volcanic rocks and plutonic rocks Fresh lemonade physical or chemical change
Fresh lemonade physical or chemical change Fresh lemonade physical or chemical change
Fresh lemonade physical or chemical change Carbonation of rock
Carbonation of rock Sub aerial weathering
Sub aerial weathering Weathering rocks
Weathering rocks What are the two types of weathering
What are the two types of weathering Compare and contrast mechanical and chemical weathering
Compare and contrast mechanical and chemical weathering Compare and contrast mechanical and chemical weathering
Compare and contrast mechanical and chemical weathering The rate of weathering depends upon the area's ____
The rate of weathering depends upon the area's ____ Weathering erosion
Weathering erosion Mechanical and chemical weathering venn diagram
Mechanical and chemical weathering venn diagram Difference between weathering and erosion
Difference between weathering and erosion Mechanical and chemical weathering venn diagram
Mechanical and chemical weathering venn diagram Mechanical and chemical weathering
Mechanical and chemical weathering Mechanical digestion and chemical digestion venn diagram
Mechanical digestion and chemical digestion venn diagram Causes of weathering
Causes of weathering Mechanical and chemical weathering
Mechanical and chemical weathering Mechanical and chemical weathering venn diagram
Mechanical and chemical weathering venn diagram Time factor in soil formation
Time factor in soil formation