Chemical Reactions Chapter 11 Writing chemical equations To





- Slides: 14

Chemical Reactions Chapter 11

Writing chemical equations To write a word equation: write names of reactants left of the arrow separated by plus signs; write names of products right of the arrow separated by plus signs. Hydrogen + oxygen water

Skeleton equation Chemical equation that does not indicate amounts of the reactants and products. Not balanced! H 2(g) + O 2(g) H 2 O(l) Catalyst is a substance that speeds up the reaction but is not consumed in the reaction. Neither reactant or product and written above the arrow.

Balancing equations Coefficients: small whole numbers that are placed in front of the formulas in an equation in order to balance it. A balanced equation is where each side of the equation has equal numbers of atoms of each element and mass is conserved.

Balancing equations First write the skeleton equation Then use coefficients to balance the equation so that it obeys the law of conservation of mass. Never change subscripts. Simplify if possible

Chemical reactions The five general types of reactions are: Combination Decomposition Single-replacement Double-replacement Combustion *Chemical change occurs with all of them!
Combination Reactions Two or more substances react to form a single new substance. 2 Mg(s) + O 2(g) 2 Mg. O(s)

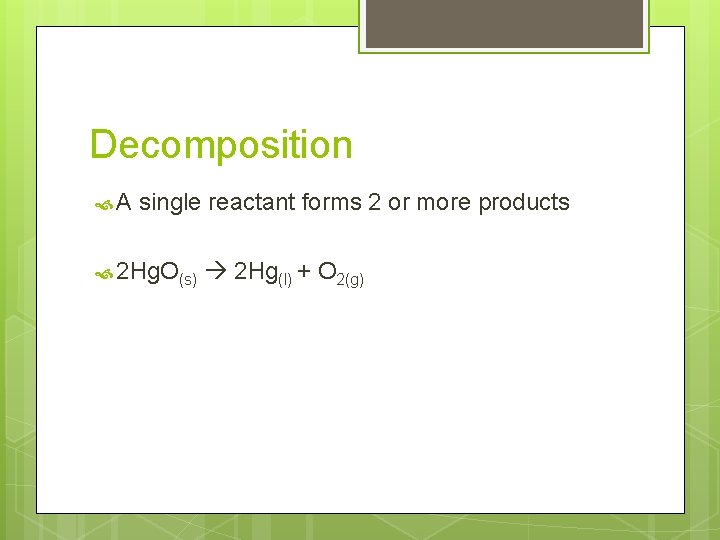
Decomposition A single reactant forms 2 or more products 2 Hg. O(s) 2 Hg(l) + O 2(g)

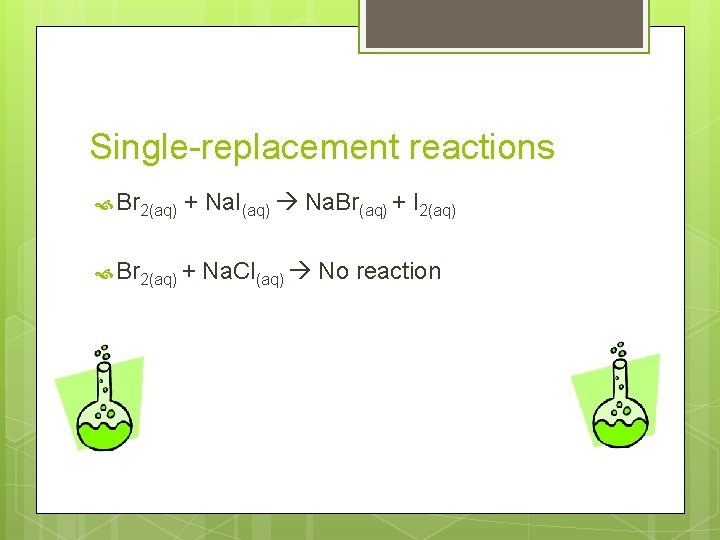
Single-Replacement Reactions One element replaces a second element in a compound. Both the reactants and products have a single element and a compound. Activity series: lists metals in order of decreasing reactivity. Page 333 A reactive metal will replace any metal listed below it in the activity series. A reactive halogen will replace any halogen below it.

Single-replacement reactions Br 2(aq) + Na. I(aq) Na. Br(aq) + I 2(aq) Br 2(aq) + Na. Cl(aq) No reaction

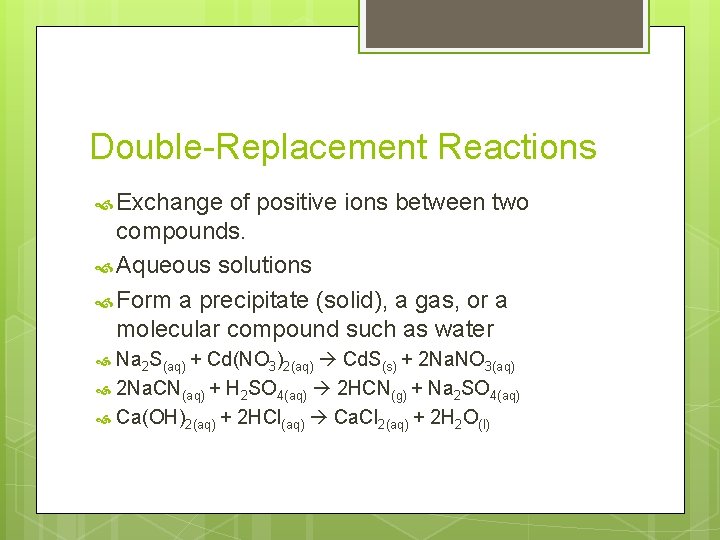
Double-Replacement Reactions Exchange of positive ions between two compounds. Aqueous solutions Form a precipitate (solid), a gas, or a molecular compound such as water Na 2 S(aq) + Cd(NO 3)2(aq) Cd. S(s) + 2 Na. NO 3(aq) 2 Na. CN(aq) + H 2 SO 4(aq) 2 HCN(g) + Na 2 SO 4(aq) Ca(OH)2(aq) + 2 HCl(aq) Ca. Cl 2(aq) + 2 H 2 O(l)

Combustion Reaction An element or a compound reacts with oxygen often producing energy in the form of heat and light. Always involves oxygen as a reactant. The other reactant is usually a hydrocarbon (compound composed of carbons and hydrogens). Products are carbon dioxide and water for complete combustion.

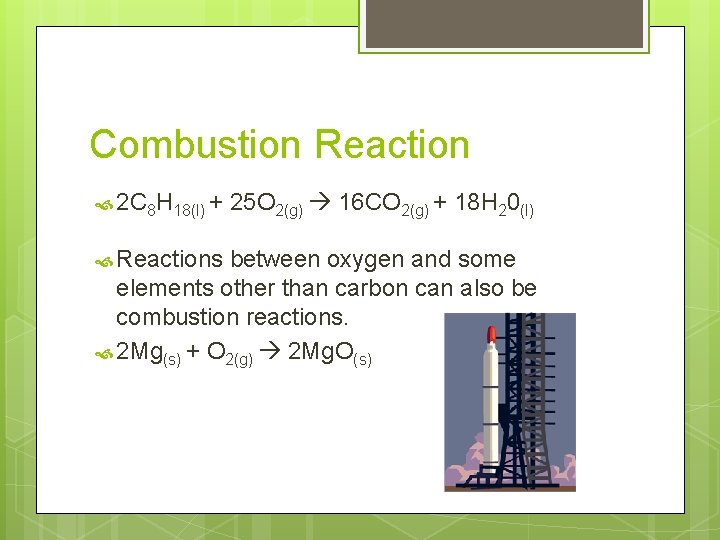
Combustion Reaction 2 C 8 H 18(l) + Reactions 25 O 2(g) 16 CO 2(g) + 18 H 20(l) between oxygen and some elements other than carbon can also be combustion reactions. 2 Mg(s) + O 2(g) 2 Mg. O(s)

Predicting the products of a chemical reaction The number of elements and/or compounds reacting is a good indicator of possible reaction type and thus possible products. Figure 11. 10 on page 338 -339
 Chapter 18 chemical reactions balancing chemical equations
Chapter 18 chemical reactions balancing chemical equations Chemical reactions section 2 classifying chemical reactions
Chemical reactions section 2 classifying chemical reactions Section 2 classifying chemical reactions worksheet answers
Section 2 classifying chemical reactions worksheet answers Chapter 8 review chemical equations and reactions
Chapter 8 review chemical equations and reactions Chapter 8 section 1 chemical equations and reactions
Chapter 8 section 1 chemical equations and reactions Chapter 8 review chemical equations and reactions
Chapter 8 review chemical equations and reactions Synthesis reaction
Synthesis reaction Types of reactions
Types of reactions Lesson 68 toxic reactions chemical equations answer key
Lesson 68 toxic reactions chemical equations answer key Hcl and sodium hydrogen carbonate
Hcl and sodium hydrogen carbonate Unit 5 chemical equations and reactions
Unit 5 chemical equations and reactions Toxic reactions chemical equations
Toxic reactions chemical equations Chapter 10 chemical reactions
Chapter 10 chemical reactions Chapter 9 chemical reactions test answers
Chapter 9 chemical reactions test answers Chemical reactions section 1 chemical changes
Chemical reactions section 1 chemical changes