CHEMICAL Equations WORD EQUATION Potassium metal Reactants are




- Slides: 10

CHEMICAL Equations

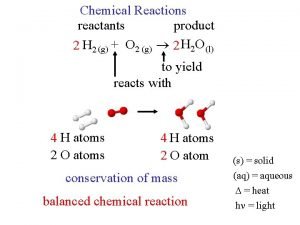
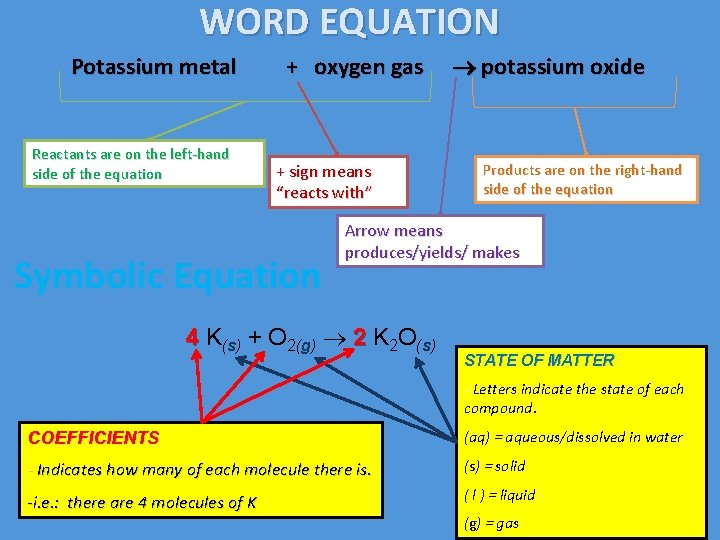
WORD EQUATION Potassium metal Reactants are on the left-hand side of the equation + oxygen gas + sign means “reacts with” Symbolic Equation potassium oxide Products are on the right-hand side of the equation Arrow means produces/yields/ makes 4 K(s) + O 2(g) 2 K 2 O(s) STATE OF MATTER - Letters indicate the state of each compound. COEFFICIENTS (aq) = aqueous/dissolved in water - Indicates how many of each molecule there is. (s) = solid -i. e. : there are 4 molecules of K ( l ) = liquid (g) = gas

Conservation of Mass - total mass of the products is always equal to the total mass of the reactant

Balancing Equations 4 K + O 2 2 K 2 O • Law of conservation of mass says you must have equal number of atoms on the reactant side as there is on the product side. • Compare reactants with products, which atoms are unbalanced? ? • Note: If there is no number as a coefficient than it is 1 • You can only change the coefficients (in front of each substance) and not subscripts behind!

TIPS for Balancing Equations • Balance chemical equations tips – Count all atoms & recount each time – Polyatomic ions (such as SO 42–) can often be balanced as a whole group – Balance the H’s 2 nd last – Balance O’s Last – Always double-check after you think you are finished!

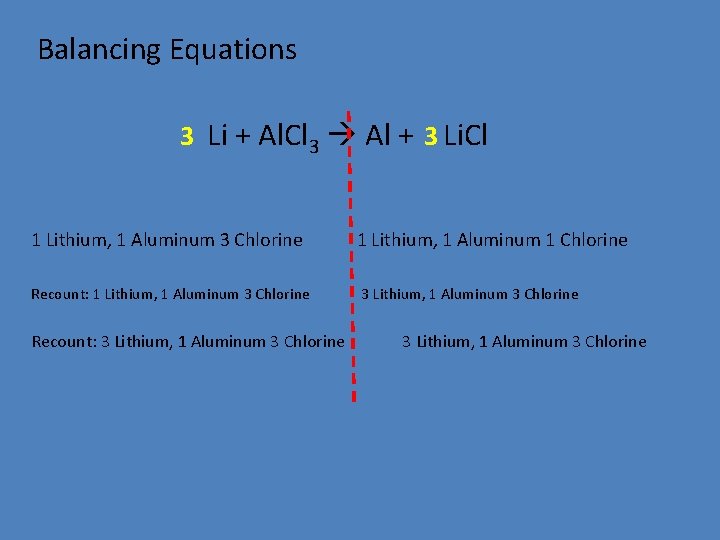
Balancing Equations 3 Li + Al. Cl 3 Al + 3 Li. Cl 1 Lithium, 1 Aluminum 3 Chlorine 1 Lithium, 1 Aluminum 1 Chlorine Recount: 1 Lithium, 1 Aluminum 3 Chlorine 3 Lithium, 1 Aluminum 3 Chlorine Recount: 3 Lithium, 1 Aluminum 3 Chlorine

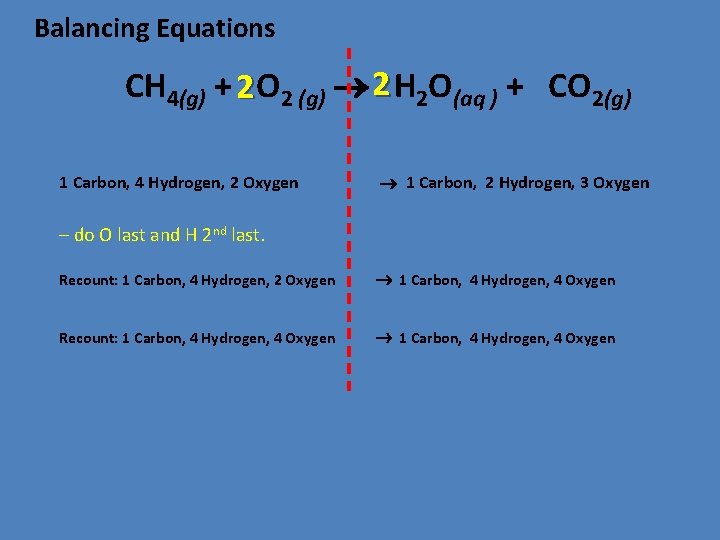
Balancing Equations CH 4(g) + 2 O 2 (g) 2 H 2 O(aq ) + CO 2(g) 1 Carbon, 4 Hydrogen, 2 Oxygen 1 Carbon, 2 Hydrogen, 3 Oxygen – do O last and H 2 nd last. Recount: 1 Carbon, 4 Hydrogen, 2 Oxygen 1 Carbon, 4 Hydrogen, 4 Oxygen Recount: 1 Carbon, 4 Hydrogen, 4 Oxygen

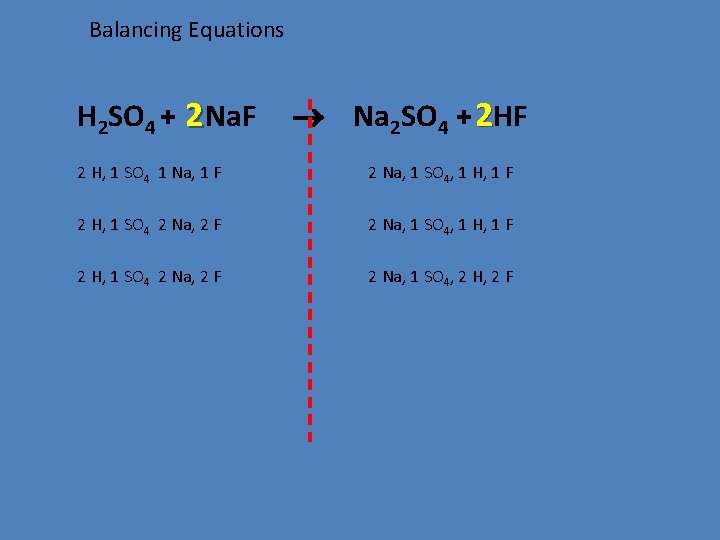
Balancing Equations H 2 SO 4 + 2 Na. F Na 2 SO 4 + 2 HF 2 H, 1 SO 4 1 Na, 1 F 2 Na, 1 SO 4, 1 H, 1 F 2 H, 1 SO 4 2 Na, 2 F 2 Na, 1 SO 4, 2 H, 2 F

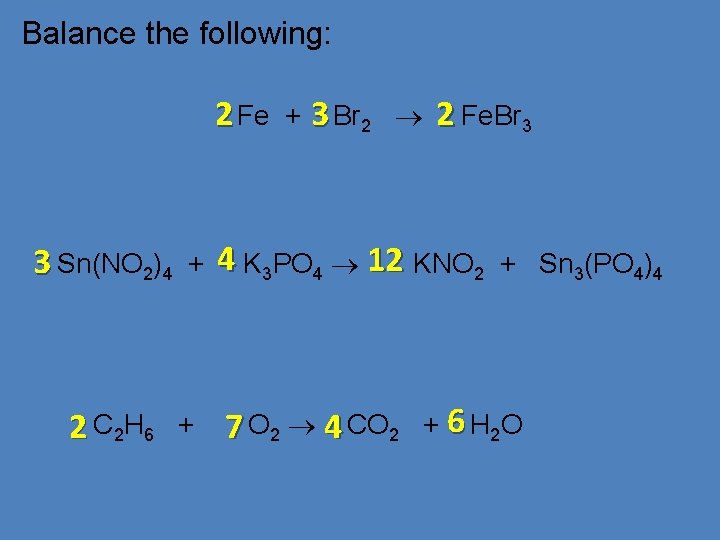
Balance the following: 2 Fe 3 Sn(NO 2)4 2 C 2 H 6 + + + 3 Br 2 2 Fe. Br 3 4 K 3 PO 4 12 KNO 2 7 O 2 4 CO 2 + Sn 3(PO 4)4 + 6 H 2 O

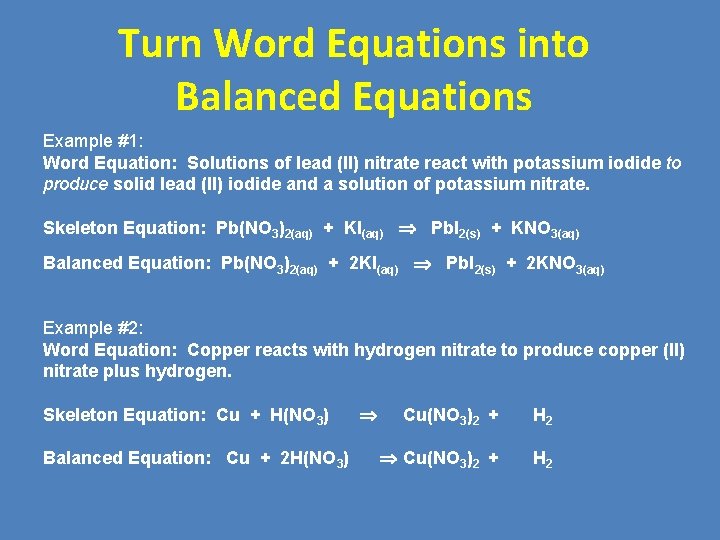
Turn Word Equations into Balanced Equations Example #1: Word Equation: Solutions of lead (II) nitrate react with potassium iodide to produce solid lead (II) iodide and a solution of potassium nitrate. Skeleton Equation: Pb(NO 3)2(aq) + KI(aq) Pb. I 2(s) + KNO 3(aq) Balanced Equation: Pb(NO 3)2(aq) + 2 KI(aq) Pb. I 2(s) + 2 KNO 3(aq) Example #2: Word Equation: Copper reacts with hydrogen nitrate to produce copper (II) nitrate plus hydrogen. Skeleton Equation: Cu + H(NO 3) Balanced Equation: Cu + 2 H(NO 3) Cu(NO 3)2 + H 2
 Antigentest åre
Antigentest åre Translating chemical equations
Translating chemical equations Chemical reactions reactants and products
Chemical reactions reactants and products Reactants and products
Reactants and products Are kc and kp equal
Are kc and kp equal Chapter 19 chemical reactions simple word equations
Chapter 19 chemical reactions simple word equations How to type chemical equation in word
How to type chemical equation in word Chlorine + potassium bromide
Chlorine + potassium bromide Potassium chloride silver nitrate
Potassium chloride silver nitrate Lead iodide and potassium nitrate
Lead iodide and potassium nitrate Venn diagram states of matter
Venn diagram states of matter