Chem 250 129 Lecture Announcements I A 122















- Slides: 28

Chem. 250 – 12/9 Lecture

Announcements - I A. 12/2 assigned homework problem solutions also posted (soon) B. Turn in Term Project Papers C. Last Homework Assignment Chapter 16: 1, 4, 5, 6, 7, 8, 14, 15, 16 (No need to turn in; Solutions to be posted by Friday)

Announcements - II D. Final Exam 1. Wed. , 12/16, 5: 15 to 7: 15 pm 2. Exam will be 100 points and is NOT cumulative. 3. There will be both concept type questions (multiple choice or fill in the blank) and problems/short essay.

Toxicity (Ch. 16) - Overview A. B. C. D. Exposure Dose/Response Fate of Toxic Substances in the Body Types of Effects

Toxicity - Exposure • The degree to which a toxic substance will cause health problems will depend on the following conditions: – – – Concentration of substance Form of substance Route of entry to body Time period in which body is exposed Body’s “reaction” to toxic substance (only above are considered exposure)

Toxicity - Exposure Form of substance: - The form of the substance will affect how toxic compounds pass through barriers (skin, GI tract, airways) Some examples: - mercury (methyl mercury vs. inorganic mercury) - aerosol particles (coarse vs. fine; acidic vs. neutral) - compounds in specific solvents (e. g. dimethylsulfoxide vs. water) Any form that makes barrier passage easier increases toxicity

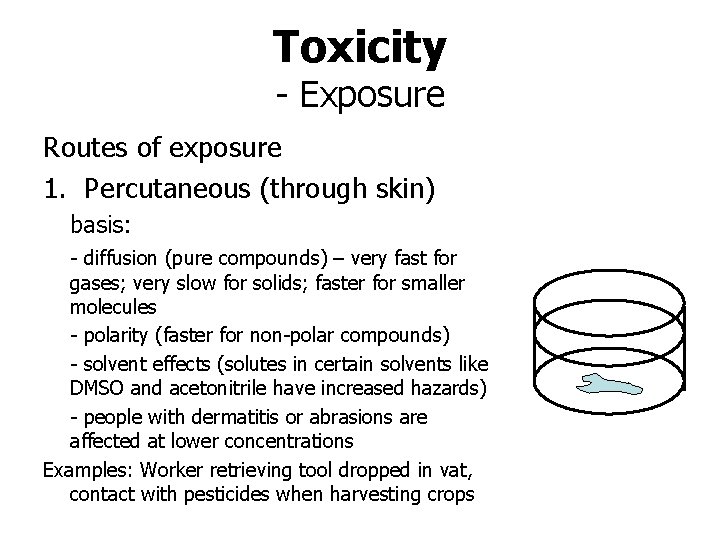
Toxicity - Exposure Routes of exposure 1. Percutaneous (through skin) basis: - diffusion (pure compounds) – very fast for gases; very slow for solids; faster for smaller molecules - polarity (faster for non-polar compounds) - solvent effects (solutes in certain solvents like DMSO and acetonitrile have increased hazards) - people with dermatitis or abrasions are affected at lower concentrations Examples: Worker retrieving tool dropped in vat, contact with pesticides when harvesting crops

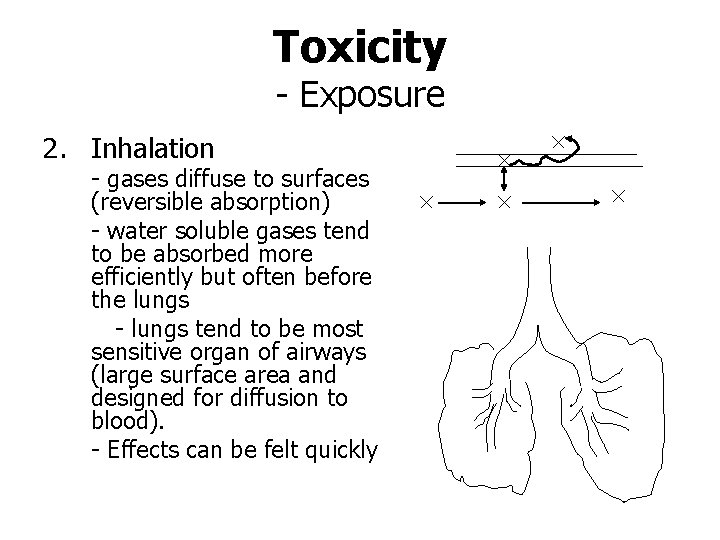
Toxicity - Exposure 2. Inhalation - gases diffuse to surfaces (reversible absorption) - water soluble gases tend to be absorbed more efficiently but often before the lungs - lungs tend to be most sensitive organ of airways (large surface area and designed for diffusion to blood). - Effects can be felt quickly

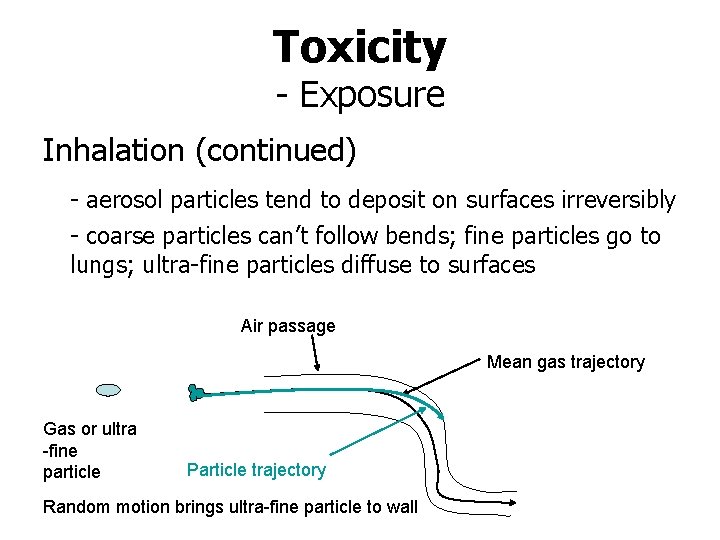
Toxicity - Exposure Inhalation (continued) - aerosol particles tend to deposit on surfaces irreversibly - coarse particles can’t follow bends; fine particles go to lungs; ultra-fine particles diffuse to surfaces Air passage Mean gas trajectory Gas or ultra -fine particle Particle trajectory Random motion brings ultra-fine particle to wall

Toxicity - Exposure (Inhalation) Airways have defense mechanisms (mucous and cilia to remove particles) Toxic response can be due to passage to blood and rest of body (pulmonary problems) or to lung cell damage (e. g. asbestos) People with asthma can suffer at much lower doses than others Respiration affects exposure. Heavy work or exercise in poor air quality can be harmful.

Toxicity - Exposure 3. Oral - Absorption occurs in the gastrointestinal tract (GI tract) - Less polar compounds are preferentially absorbed - For acids and bases, absorption of non-ionized species is much faster - Absorption also depends on p. H of organ (~2 for stomach vs. ~6 for intestines) - Exposure can occur from contaminated foods (e. g. with pesticide or natural toxin present) or from incidental ingestion (e. g. lead in toys or paint flakes, chemistry lab student eating cheetos, my son spraying my toothbrush with insect repellent)

Toxicity - Exposure • Minimization of Exposure – Use of protective equipment: • Gloves, protective clothes, goggles (dermal) • Masks or respirators (inhalation) – Use of equipment to keep chemicals out of contact • Use of fume hoods • Segregation of work space into different regions

Toxicity - Some questions I 1. 2. 3. 4. Besides concentrations, list three other factors that affect how toxic a substance is In which form is capsaicin (a moderate sized compound of weak polarity) absorbed better into the skin? a) As solid powder b) in water c) in acetonitrile Which form of lead in the environment is most likely to cause acute exposure problems: a) solid lead, b) tetraethyl lead, c) lead sulfate (moderately soluble in water), d) lead phosphate (very insoluble in water) A researcher found that SO 2 inhalation causes more damage after patients drank lemonade or other acidic drinks. Explain why?

Toxicity - Some questions II 4. 5. 6. A lawyer argues that arsenic in aerosols emitted from an incinerator has the same concentration as aerosols produced from soil dust in natural dust storms. Does this make the incinerator safe? Why or why not? What else should a safety expert know? Which part of the GI tract would the following compound be absorbed in: a) nitrous acid (p. Ka = 3. 1), b) aniline (p. Ka of base cation = 4. 6) c) ethyl amine (p. Ka of base cation = 10. 6)? Chelating ligands like EDTA are administrated to remove toxic metals. Based on p. H considerations and on movement across the GI tract in reverse, will ligands be more effective in the stomach or intestines?

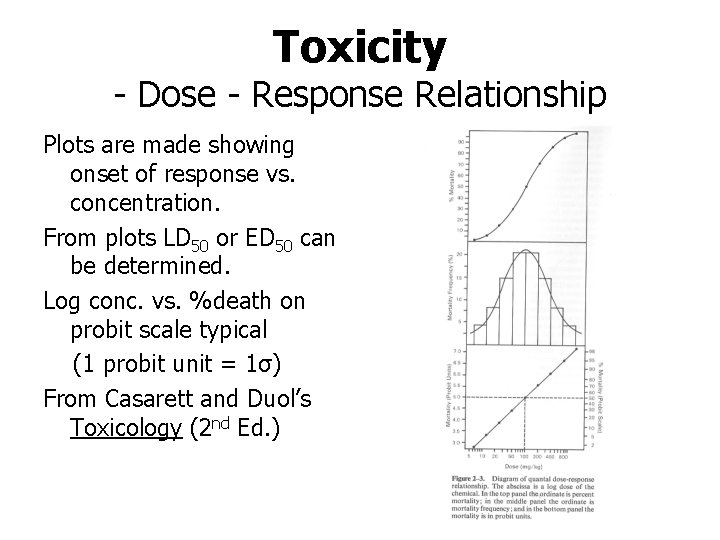
Toxicity - Dose - Response Relationship The concentration of a toxic substance in a body (e. g. mg of toxin per kg body weight) is related to the response of the body Numerous responses are possible (e. g. from inhibition of specific enzyme to organ failure to death) Common responses examined are effective dose (ED), toxic dose (TD) and lethal dose (LD) Relationship between dose and response is generally better behaved for acute toxicity

Toxicity - Dose - Response Relationship Plots are made showing onset of response vs. concentration. From plots LD 50 or ED 50 can be determined. Log conc. vs. %death on probit scale typical (1 probit unit = 1σ) From Casarett and Duol’s Toxicology (2 nd Ed. )

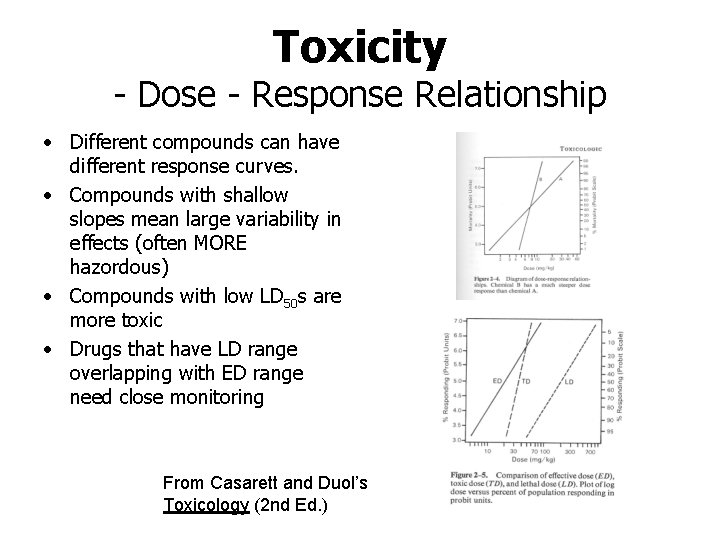
Toxicity - Dose - Response Relationship • Different compounds can have different response curves. • Compounds with shallow slopes mean large variability in effects (often MORE hazordous) • Compounds with low LD 50 s are more toxic • Drugs that have LD range overlapping with ED range need close monitoring From Casarett and Duol’s Toxicology (2 nd Ed. )

Toxicity - Redistribution in Body • Movement to target organs/tissue (e. g. Hg to nerve tissue) • Storage Tissue/Organs – fats (for compounds with large Kows) – bones (for certain inorganic compounds) • Organs with High Concentrations: – Liver + Kidneys – Normal because these organs used for chemical transformation (liver) or excretion (kidney)

Toxicity - Fate of Substances Toxic compounds in the body can be: 1) excreted or 2) transformed More water soluble compounds tend to be excreted more quickly, while lipid soluble compounds can have long lifetimes (months) A common transformation is enzymatic oxidation in the liver (particularly for lipid soluble compounds) Oxidation can lead to decreases or increases in toxicity, but usually leads to faster excretion due to increase in polarity

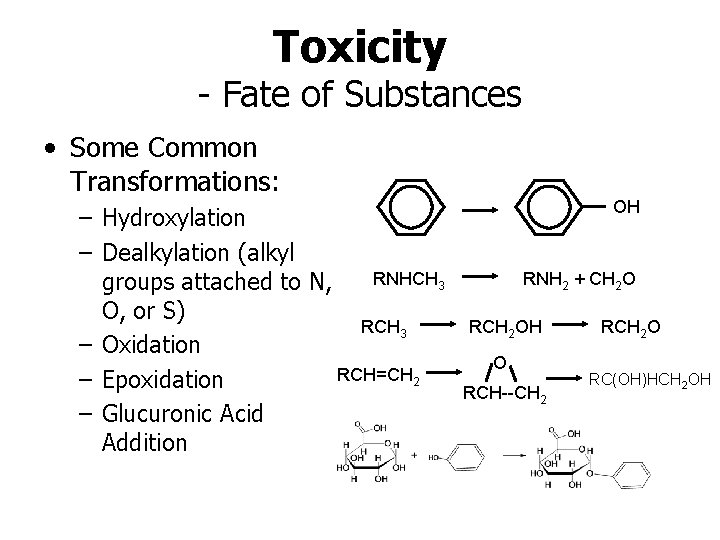
Toxicity - Fate of Substances • Some Common Transformations: – Hydroxylation – Dealkylation (alkyl RNHCH 3 groups attached to N, O, or S) RCH 3 – Oxidation RCH=CH 2 – Epoxidation – Glucuronic Acid Addition OH RNH 2 + CH 2 O RCH 2 OH O RCH--CH 2 RCH 2 O RC(OH)HCH 2 OH

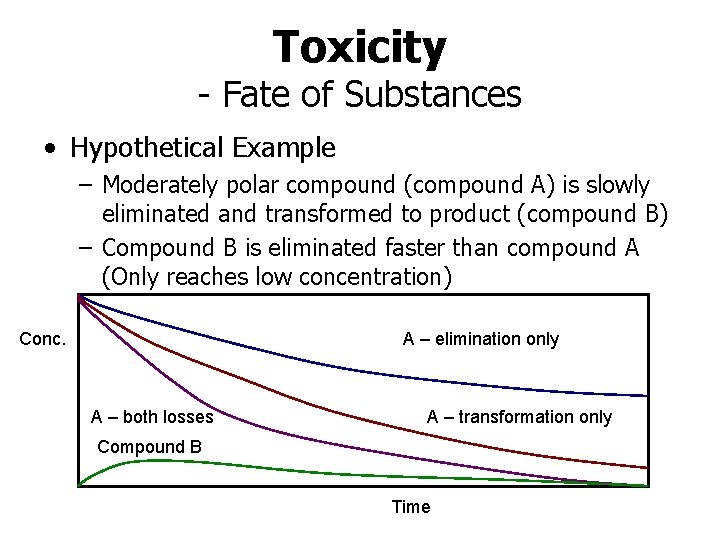
Toxicity - Fate of Substances • Hypothetical Example – Moderately polar compound (compound A) is slowly eliminated and transformed to product (compound B) – Compound B is eliminated faster than compound A (Only reaches low concentration) Conc. A – elimination only A – both losses A – transformation only Compound B Time

Toxicity - Removal of Substances • Polar/Water soluble compounds are often eliminated through urination • Volatile compounds can be eliminated by exhalation • Less polar compounds can be removed from liver through bile (goes back to GI tract) • Other routes – GI tract – Sweat

Toxicity - Biotracer Studies • Exposure is often difficult to assess accurately • An alternative approach is to directly measure concentrations of toxin or metabolites in urine • Factors affecting exposure then can be studied by comparing environmental concentrations with detected amounts

Toxicity - Types of Effects • Acute Effects – exposure period is typically short but intense – effects occur soon after exposure – effects may be reversible or irreversible • Chronic Effects – exposure period is typically over a period of time – effects generally take time to develop (e. g. cancer) – may occur from build up of product (e. g. calcium oxalate from ingestion of ethylene glycol) – may result from body’s reaction to toxin (e. g. build up of scar tissue) – may occur for low probability effects (mutation of DNA) – determining relationship is more difficult

Toxicity - Chronic Effects • Teratogens – Compounds that affect normal development of fetuses • Mutagens – Cause changes to DNA sequences • Carcinogens – Compounds that can lead to the development of cancer

Toxicity - Chronic Effects • Effects of Carcinogens – Most carcinogens (or their metabolites) can react with and alter DNA – Carcinogens may react with base pairs (e. g. aromatic compounds) or with sugar or phosphate parts – Many changes to DNA sequence have little effect (may kill cell or have minor effects) – Changes to DNA can affect normal mechanisms to restrict cell growth – Proliferation of deformed cells is what leads to problems with cancer

Toxicity - Chronic Effects • Determination of Carcinogens – Epidemiological Studies (comparison of environmental exposure with effects on population) – Animal Tests (e. g. feeding compounds to rats or mice) – Ames Test (bacterial screening test for mutagens)

Toxicity - More questions I 1. 2. 3. 4. 5. The LD 50 for paraquat is about 100 mg/kg. Estimate the amount of paraquat that would need to be ingested by a “typical” 50 kg woman to just die from it. Why might it be prudent to limit exposures of toxic compounds to several orders of magnitude under the LD 50? What are possible metabolic products of CH 2=CHCH 2 OCH 3? If a person is exposed to a toxic substance over a short time period, how might the timeline of effects vary depending on a) compound polarity and b) toxicity of compound vs. metabolites? Regulations often limit toxic substances through a) absolute concentration limits and b) time averaged concentration limits. Why would two separate limits be useful? What are the two limits protecting against?
 Nec article 810
Nec article 810 Nec 250 122
Nec 250 122 Fahrenheit 451 burning bright
Fahrenheit 451 burning bright Pvu market cap
Pvu market cap Kayl announcements
Kayl announcements R/announcements
R/announcements General announcements
General announcements David ritthaler
David ritthaler 01:640:244 lecture notes - lecture 15: plat, idah, farad
01:640:244 lecture notes - lecture 15: plat, idah, farad Taskj-129
Taskj-129 Rojo 3 colorante toxicidad
Rojo 3 colorante toxicidad 300 - 129
300 - 129 Signing naturally 3:11
Signing naturally 3:11 Art 129 cst
Art 129 cst Ecs 129
Ecs 129 Round 763 to the nearest hundred
Round 763 to the nearest hundred Psalm 68 tpt
Psalm 68 tpt Eecs 122
Eecs 122 Mandatos negativos con tú (p. 122)
Mandatos negativos con tú (p. 122) Frc pneumatics diagram
Frc pneumatics diagram Psalm 122:1-9
Psalm 122:1-9 122+105
122+105 Ee 122
Ee 122 Ece 122
Ece 122 Ece 122
Ece 122 Psalm 22:6-8
Psalm 22:6-8 Psalm 122 passion translation
Psalm 122 passion translation Hyponomy
Hyponomy Dns example
Dns example