Chapter 7 Chemical Reactions Chemical Reactions Physical change












- Slides: 20

Chapter 7 - Chemical Reactions

Chemical Reactions • Physical change is when the chemical composition of the substance remains constant, while for chemical change, the chemical composition does not remain constant. • EVIDENCE OF CHEMICAL REACTION • A gas is released in form of 1) bubbles 2) fizzing evidence. 3) carbon dioxide gas released. • An insoluble solid produced (precipitate) : aqueous solution is formed when a substance dissolves in water. When we add two solutions together, we may observe solid particles in solution. • Permanent color change: many chemical reactions involve a permanent color change. Some are obvious while in some, like acid/ base reaction, an indicator (a substance that change color) is used. The indicator enables us to indirectly follow a reaction that would otherwise not be visible. • A heat energy change is noted: In chemical reactions there is often a change in temperature. A reaction that releases heat is said to be Exothermic reaction. A reaction that absorbs heat is said to be Endothermic reaction.

Chemical Reactions A chemical equation describes a reaction using formulas and symbols. A (aq) + B (g) C (s) + D( l) A and B are known as REACTANTS while C and D are known as PRODUCTS. The physical states are specified as follows; aq = aqueous solution, g = gas phase, s = solid phase, l = liquid phase

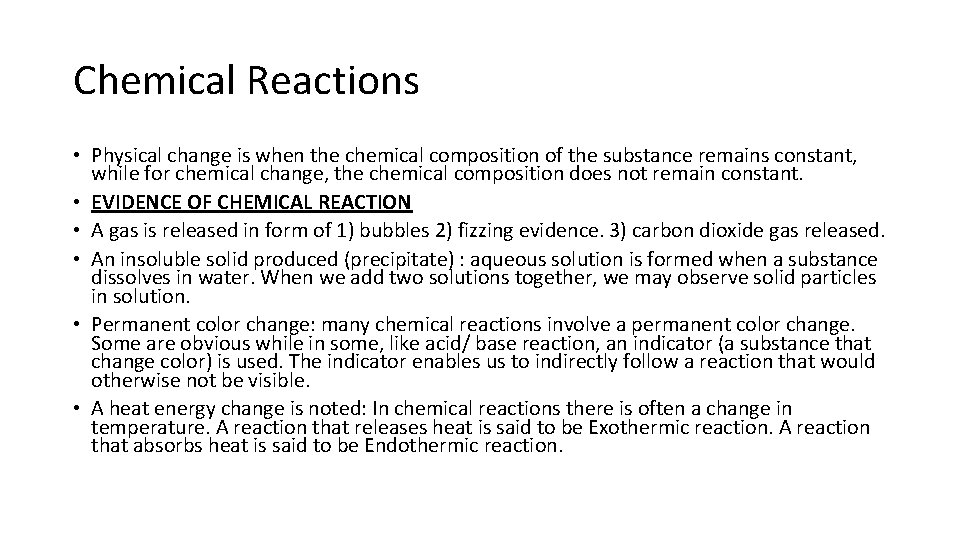
Synthesis / Combination Reaction Synthesis Reactions

Synthesis/ combination reaction • The word synthesis means to bring together separate parts into a new whole. • Description: Two reactants combine into a new compound. • Example: H 2 + O 2 H 2 O A + B A B

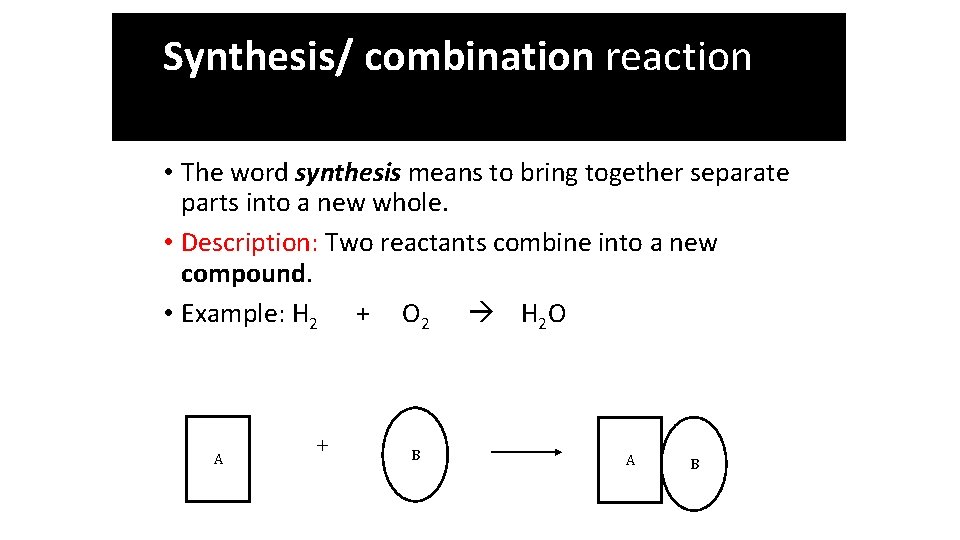
Decomposition Reactions

Decomposition reaction • The word decompose means to break a compound apart into its pieces. • Description: Decomposition reactions take a reactant and break it into two separate products. • Example: H 2 O H 2 + O 2 A B

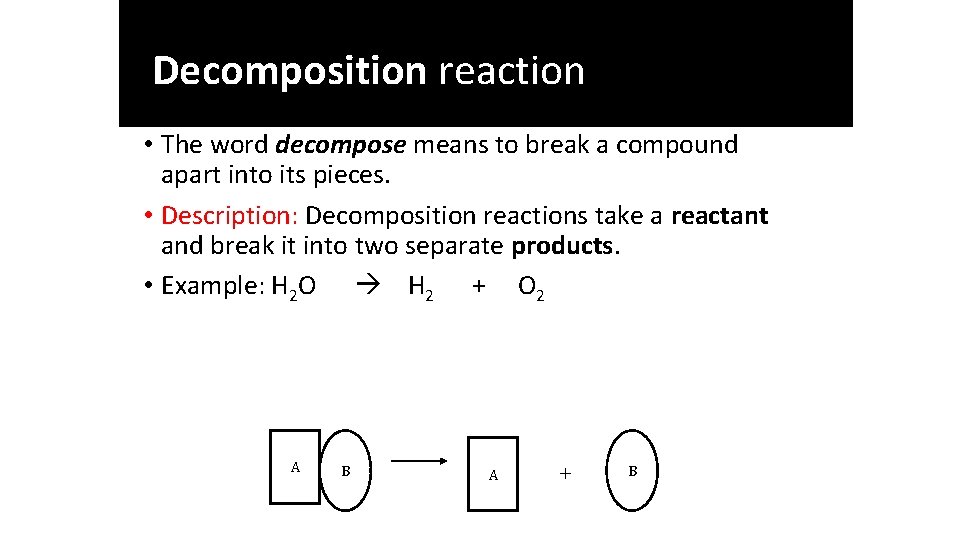
Single replacement reaction Few metals are so active that they react directly with water at room temperature- Active Metals They include group IA and group IIA metals. Specifically, Li, Na, K, Rb, Cs, Ca, Sr, and Ba react with water. The most reactive being Li> K> Ba> Sr> Ca> Na. Na(s) + 2 H 2 O(l) 2 Na. OH A B C A C B

Activity Series When a metal undergoes a single replacement reaction, it displaces another metal from a compound or aqueous solution. • A series of metals arranged in the order of their ability to undergo reaction. This series is the activity series. • Reactive metals are the metals at the top of the activity series. • Less active metals are the metals at the bottom of the activity series. • A metal can be displaced from its salt solution by an another metal which is more active.

Activity Series • If we place Zn into a solution of Ag. NO 3, then Ag is displaced because Zn is more active than Ag in the activity series: Zn(s) + 2 Ag. NO 3 Zn(NO 3)2 + 2 Ag(s) + Cu(NO 3)2(aq) NR The metals above H 2 displace hydrogen gas from water or dilute acids.

Double replacement Reaction or Metathesis Reactions • KI + Pb(NO 3)2 Pb. I 2 + KNO 3 A B C D A D B C

Precipitation Reactions or double displacement reactions A double displacement reaction in which a precipitate forms (insoluble substance) and separates from the solution. Soluble – solid dissolves in solution; (aq) is used in reaction equation. Insoluble – solid does not dissolve in solution; (s) is used in reaction equation.

Solubility Rules Ionic Compounds- Solubility Rules

Soluble/ Insoluble 1. 2. 3. 4. 5. 6. Ni. Cl 2 Ag 2 S Cs 3 PO 4 (NH 4)2 SO 4 Pb. Cl 2 Ba(OH)2

Double displacement reactions Ex: Would a precipitate form in the following reaction? If yes, what is the formula? KOH + Pb(NO 3)2 Ex: You react Ag. NO 3 with Na. I in a well plate at your lab station. Would a precipitate form? If yes, what is the formula?

Combustion Reaction • Description: A hydrocarbon (something with C and H) reacts with oxygen to form CO 2 + H 2 O • Always have form: • Cx. Hy. Oz + O 2 CO 2 + H 2 O

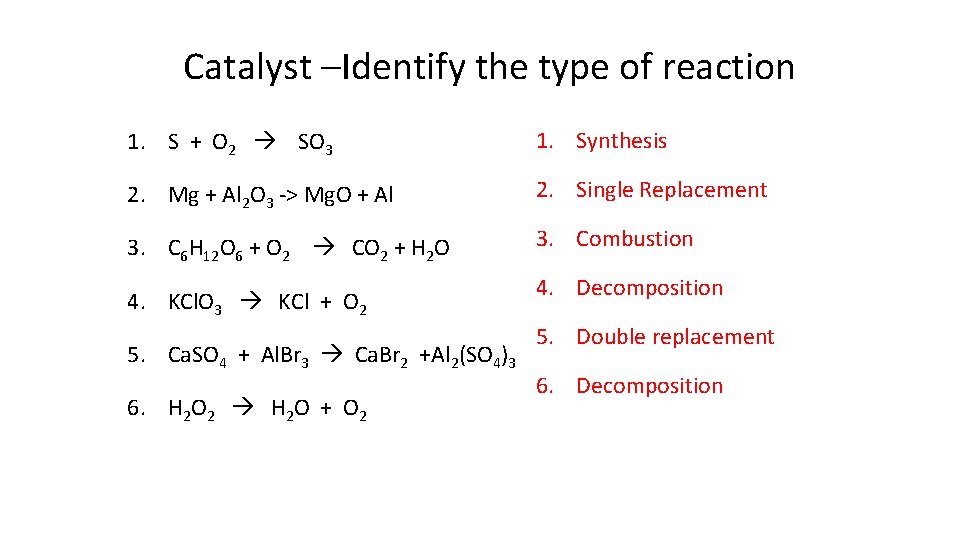
Catalyst –Identify the type of reaction 1. S + O 2 SO 3 1. Synthesis 2. Mg + Al 2 O 3 -> Mg. O + Al 2. Single Replacement 3. C 6 H 12 O 6 + O 2 CO 2 + H 2 O 3. Combustion 4. KCl. O 3 KCl + O 2 5. Ca. SO 4 + Al. Br 3 Ca. Br 2 +Al 2(SO 4)3 6. H 2 O 2 H 2 O + O 2 4. Decomposition 5. Double replacement 6. Decomposition

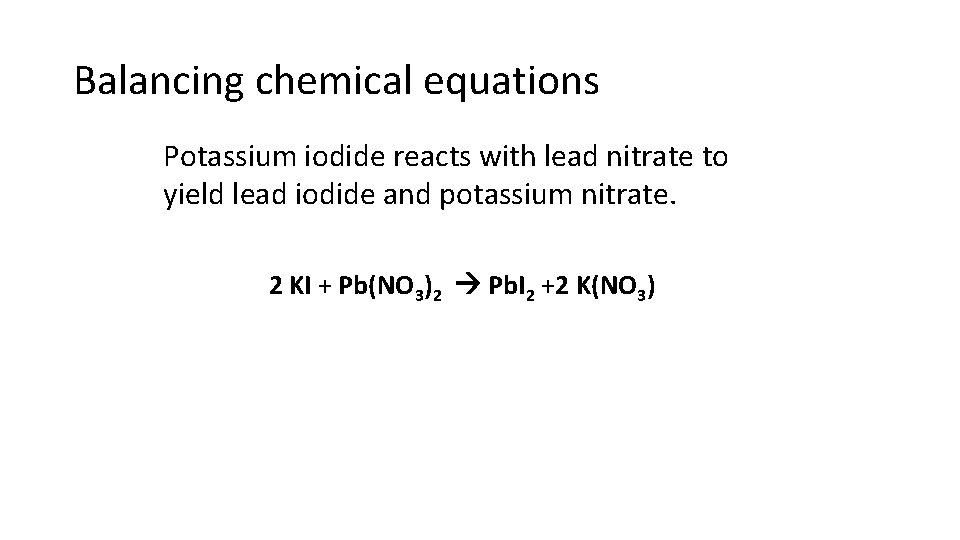
Balancing chemical equations Potassium iodide reacts with lead nitrate to yield lead iodide and potassium nitrate. 2 KI + Pb(NO 3)2 Pb. I 2 +2 K(NO 3)

Balancing Practice 1) Methane (CH 4) reacts with oxygen to form carbon dioxide and water. 2) Nitrogen gas reacts with hydrogen gas to form nitrogen trihydride.

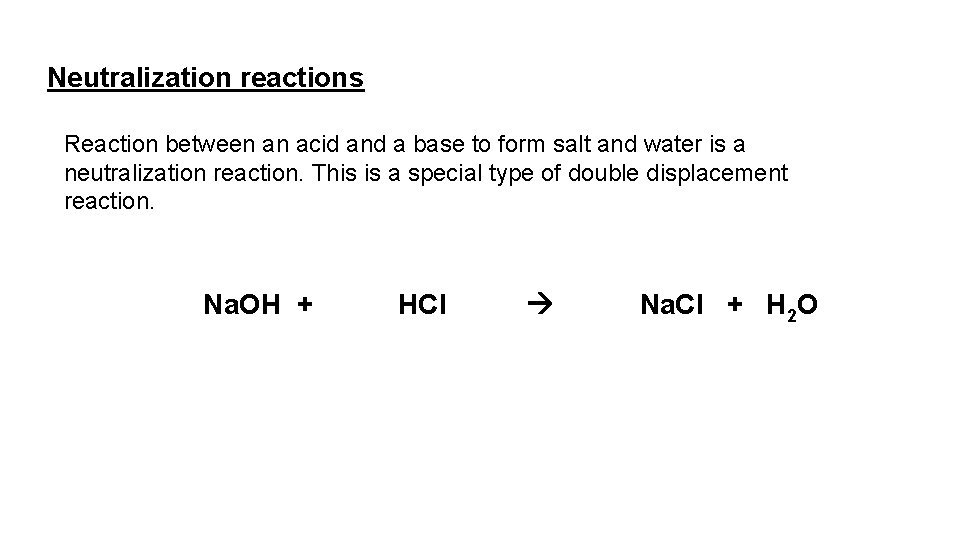
Neutralization reactions Reaction between an acid and a base to form salt and water is a neutralization reaction. This is a special type of double displacement reaction. Na. OH + HCl Na. Cl + H 2 O
 Section 2 classifying chemical reactions
Section 2 classifying chemical reactions Section 2 reinforcement classifying chemical reactions
Section 2 reinforcement classifying chemical reactions Is cutting paper a physical change
Is cutting paper a physical change Physical change chemistry
Physical change chemistry Difference between physical and chemical change
Difference between physical and chemical change Examples of physical vs chemical changes
Examples of physical vs chemical changes Spare change physical versus chemical change
Spare change physical versus chemical change Whats physical change
Whats physical change Physical change
Physical change Chemical changes in baking
Chemical changes in baking Why is chopping wood a physical change
Why is chopping wood a physical change Chapter 18 chemical reactions balancing chemical equations
Chapter 18 chemical reactions balancing chemical equations Chemical reactions section 3 reactions in aqueous solutions
Chemical reactions section 3 reactions in aqueous solutions Chapter 10 chemical reactions answer key
Chapter 10 chemical reactions answer key Chapter 9 study guide chemical reactions
Chapter 9 study guide chemical reactions Chemical reactions section 1 chemical changes
Chemical reactions section 1 chemical changes Undesirable change in the physical chemical
Undesirable change in the physical chemical Is baking cookies a physical change
Is baking cookies a physical change Is cutting aluminum foil a chemical change
Is cutting aluminum foil a chemical change Separating sand from gravel physical or chemical
Separating sand from gravel physical or chemical Urethra
Urethra





































