Chapter 3 conclusion Silicacontaining materials Xray diffraction Applications


- Slides: 15

Chapter 3 (conclusion) • • Silica-containing materials X-ray diffraction Applications of single crystals Polycrystalline materials W. R. Wilcox, Clarkson University, last revised September 17, 2013

Silica • The most common elements on earth are Si & O • Si. O 2 (silica) has 14 polymorphic crystal structures, of which quartz is the stable phase at room T & P. – http: //en. wikipedia. org/wiki/Silicon_dioxide – http: //en. wikipedia. org/wiki/Quartz • Also exists as an amorphous phase, "quartz glass" or "fused silica. " • The strong Si-O bonds lead to high melting temperatures (>1600ºC) crystobalite (stable above 1470 o. C)

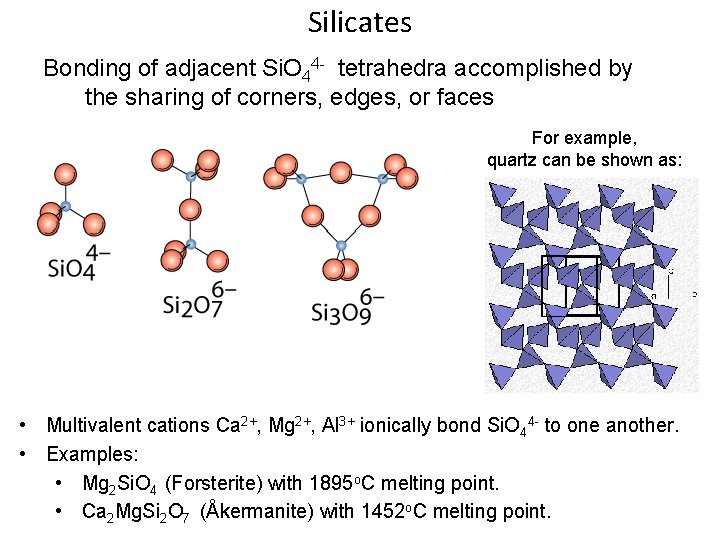
Silicates Bonding of adjacent Si. O 44 - tetrahedra accomplished by the sharing of corners, edges, or faces For example, quartz can be shown as: • Multivalent cations Ca 2+, Mg 2+, Al 3+ ionically bond Si. O 44 - to one another. • Examples: • Mg 2 Si. O 4 (Forsterite) with 1895 o. C melting point. • Ca 2 Mg. Si 2 O 7 (Åkermanite) with 1452 o. C melting point.

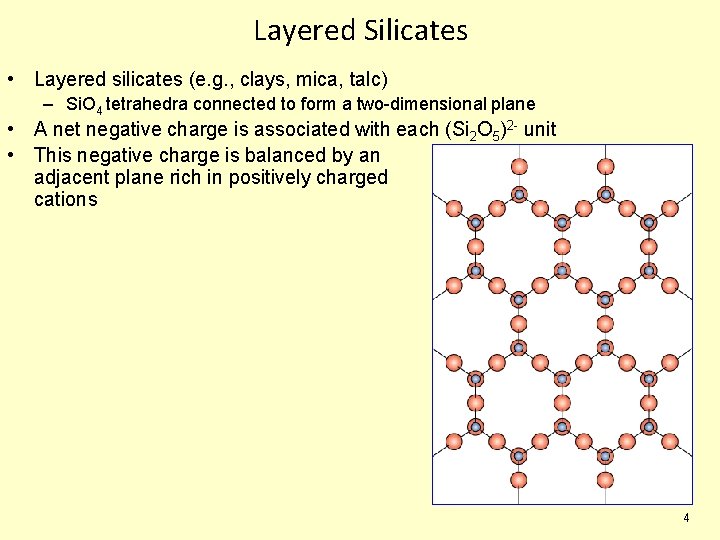
Layered Silicates • Layered silicates (e. g. , clays, mica, talc) – Si. O 4 tetrahedra connected to form a two-dimensional plane • A net negative charge is associated with each (Si 2 O 5)2 - unit • This negative charge is balanced by an adjacent plane rich in positively charged cations 4

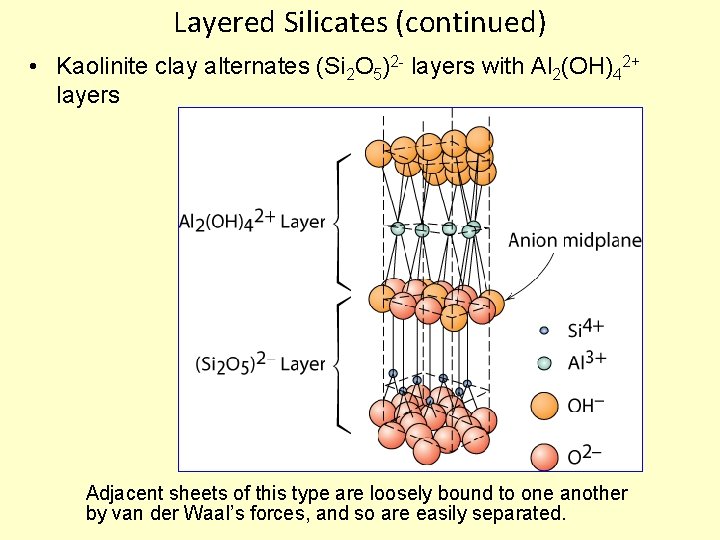
Layered Silicates (continued) • Kaolinite clay alternates (Si 2 O 5)2 - layers with Al 2(OH)42+ layers Adjacent sheets of this type are loosely bound to one another by van der Waal’s forces, and so are easily separated.

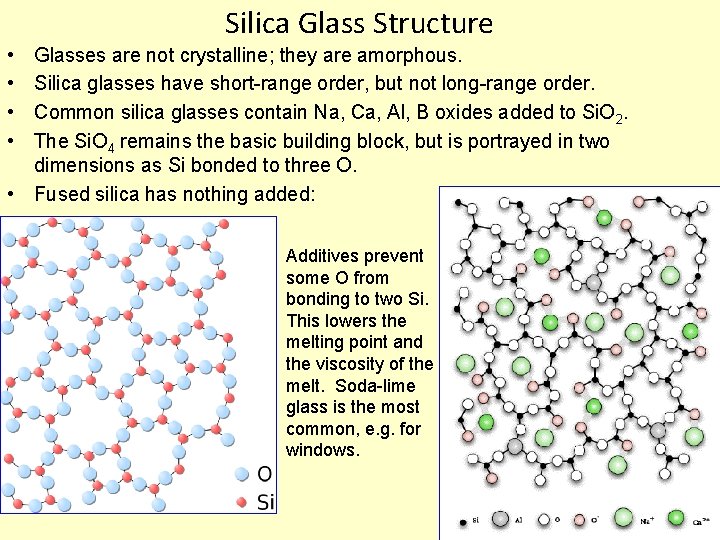
Silica Glass Structure • • Glasses are not crystalline; they are amorphous. Silica glasses have short-range order, but not long-range order. Common silica glasses contain Na, Ca, Al, B oxides added to Si. O 2. The Si. O 4 remains the basic building block, but is portrayed in two dimensions as Si bonded to three O. • Fused silica has nothing added: Additives prevent some O from bonding to two Si. This lowers the melting point and the viscosity of the melt. Soda-lime glass is the most common, e. g. for windows.

Characterization by X-Ray diffraction • An important family of characterization methods. • They utilize x-ray diffraction for various applications, e. g. , identification of a material, obtaining crystal orientation, determination of a structure, viewing defects. See, for example: http: //en. wikipedia. org/wiki/X-ray_crystallography • All techniques use a beam of x-rays of a single wavelength λ to strike a sample and a detector for the x-rays coming from the sample. • First explanation was Bragg's Law in 1913 (http: //en. wikipedia. org/wiki/Bragg%27 s_law) • Consider that crystallographic planes reflect the x-rays:

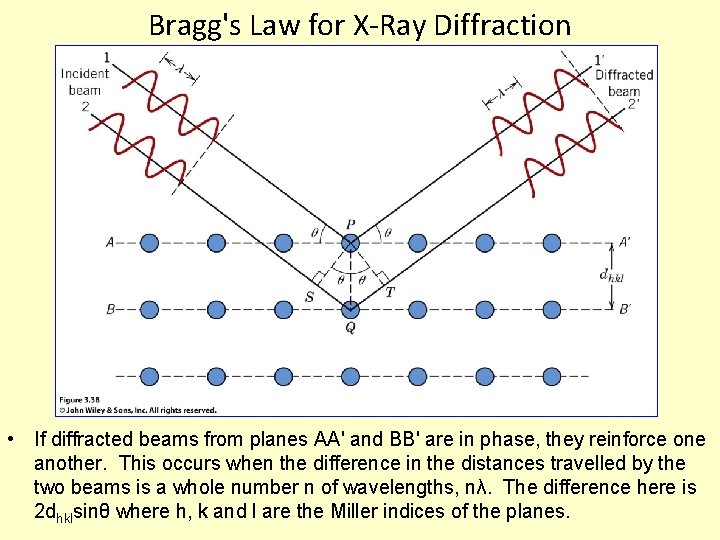
Bragg's Law for X-Ray Diffraction • If diffracted beams from planes AA' and BB' are in phase, they reinforce one another. This occurs when the difference in the distances travelled by the two beams is a whole number n of wavelengths, nλ. The difference here is 2 dhklsinθ where h, k and l are the Miller indices of the planes.

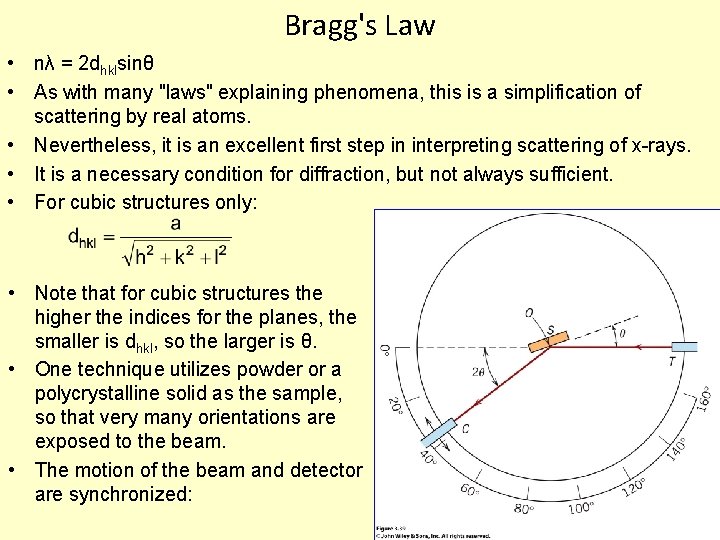
Bragg's Law • nλ = 2 dhklsinθ • As with many "laws" explaining phenomena, this is a simplification of scattering by real atoms. • Nevertheless, it is an excellent first step in interpreting scattering of x-rays. • It is a necessary condition for diffraction, but not always sufficient. • For cubic structures only: • Note that for cubic structures the higher the indices for the planes, the smaller is dhkl, so the larger is θ. • One technique utilizes powder or a polycrystalline solid as the sample, so that very many orientations are exposed to the beam. • The motion of the beam and detector are synchronized:

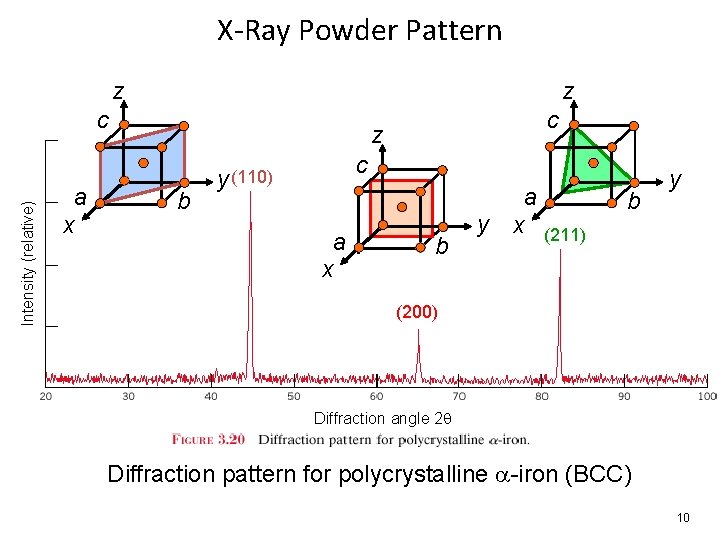
X-Ray Powder Pattern z z Intensity (relative) c a x c z b c y (110) a x b a y x (211) b y (200) Diffraction angle 2 q Diffraction pattern for polycrystalline -iron (BCC) 10

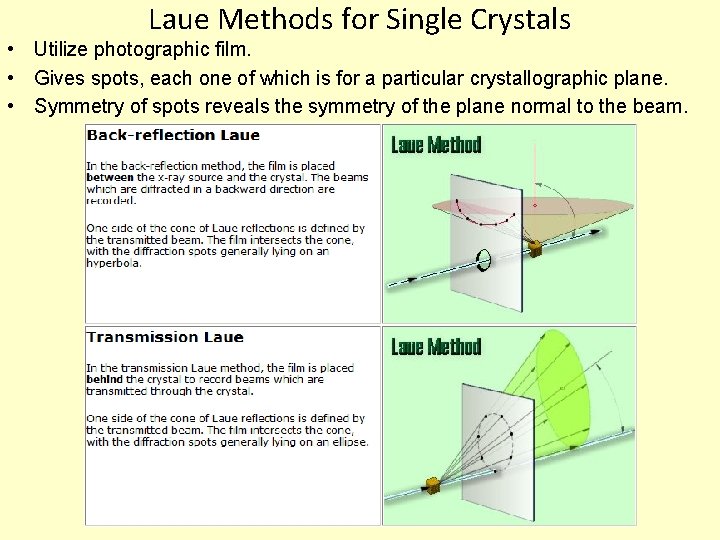
Laue Methods for Single Crystals • Utilize photographic film. • Gives spots, each one of which is for a particular crystallographic plane. • Symmetry of spots reveals the symmetry of the plane normal to the beam.

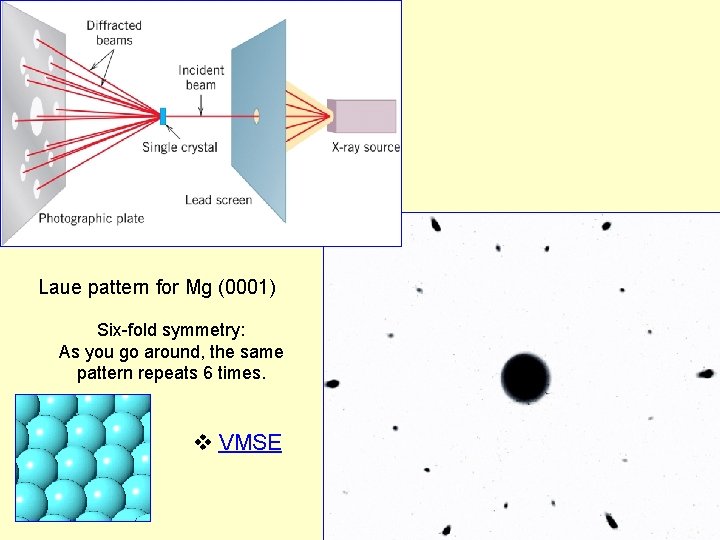
Laue pattern for Mg (0001) Six-fold symmetry: As you go around, the same pattern repeats 6 times. VMSE

http: //minerva. union. edu/jonesc/scientifi c_photos%202010. htm

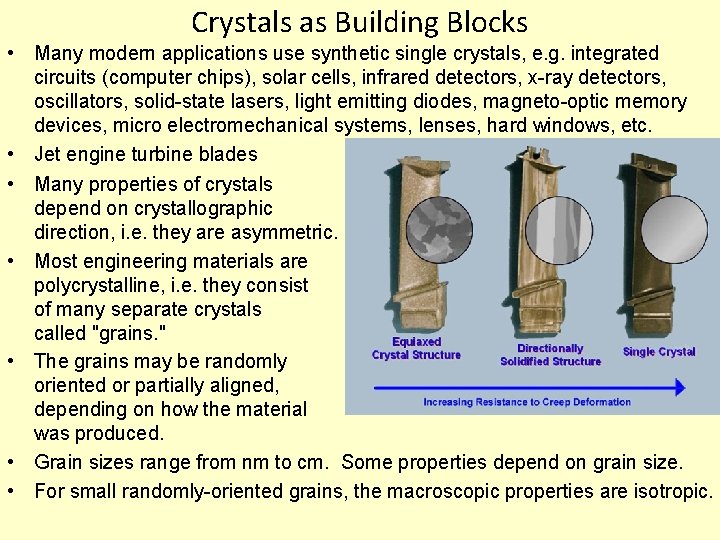
Crystals as Building Blocks • Many modern applications use synthetic single crystals, e. g. integrated circuits (computer chips), solar cells, infrared detectors, x-ray detectors, oscillators, solid-state lasers, light emitting diodes, magneto-optic memory devices, micro electromechanical systems, lenses, hard windows, etc. • Jet engine turbine blades • Many properties of crystals depend on crystallographic direction, i. e. they are asymmetric. • Most engineering materials are polycrystalline, i. e. they consist of many separate crystals called "grains. " • The grains may be randomly oriented or partially aligned, depending on how the material was produced. • Grain sizes range from nm to cm. Some properties depend on grain size. • For small randomly-oriented grains, the macroscopic properties are isotropic.

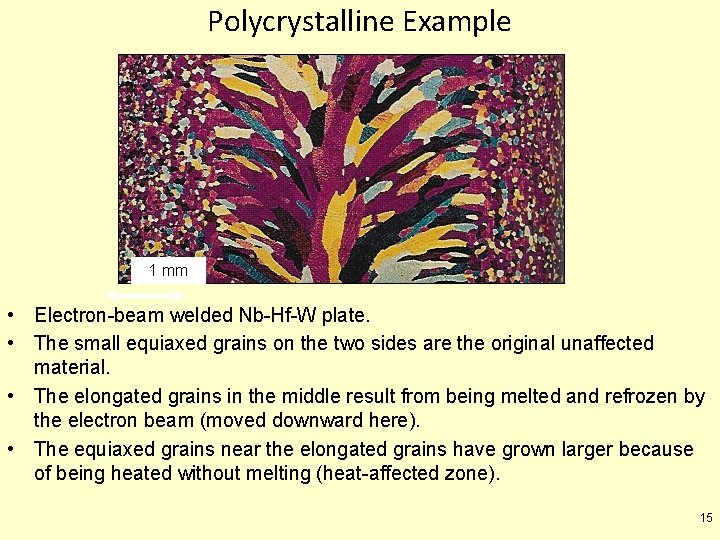
Polycrystalline Example 1 mm • Electron-beam welded Nb-Hf-W plate. • The small equiaxed grains on the two sides are the original unaffected material. • The elongated grains in the middle result from being melted and refrozen by the electron beam (moved downward here). • The equiaxed grains near the elongated grains have grown larger because of being heated without melting (heat-affected zone). 15