CHAPTER 3 ACTIVITY 5 REACTION RATES FACTORS AFFECTING


- Slides: 22

CHAPTER 3 ACTIVITY 5 REACTION RATES & FACTORS AFFECTING EQUILIBRIUM

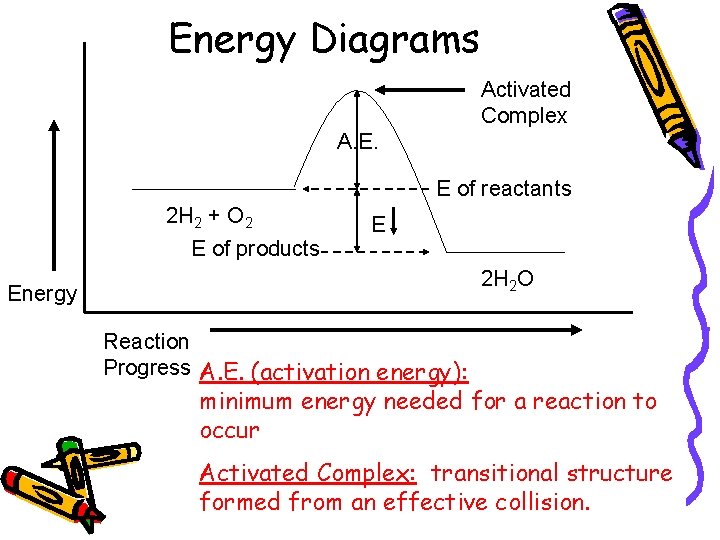
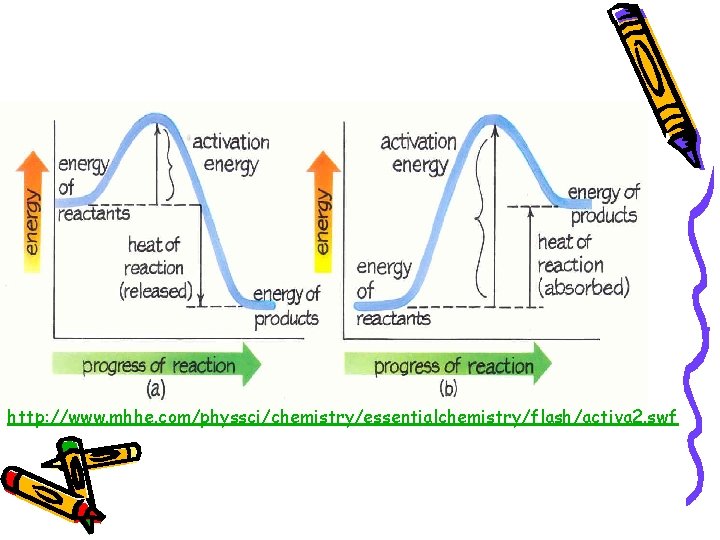
Energy Diagrams Activated Complex A. E. E of reactants 2 H 2 + O 2 E of products E 2 H 2 O Energy Reaction Progress A. E. (activation energy): minimum energy needed for a reaction to occur Activated Complex: transitional structure formed from an effective collision.

Not Every Collision Produces Products Reactants must: 1. Collide with the correct orientation 2. Have enough energy to produce products.

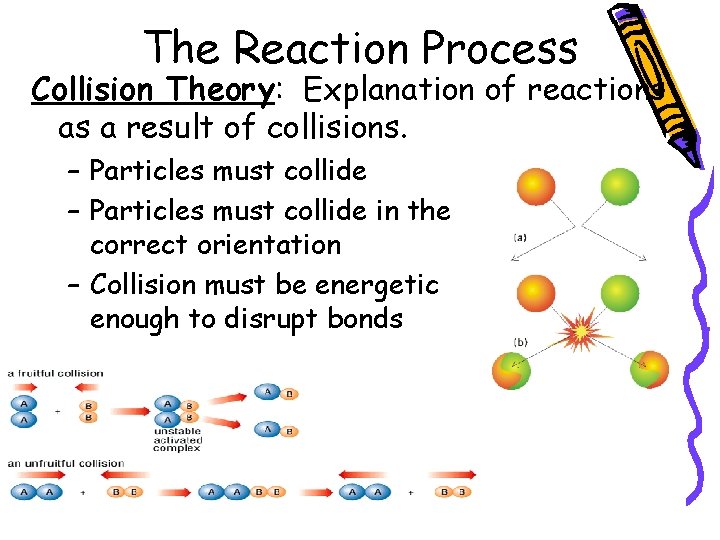
The Reaction Process Collision Theory: Explanation of reactions as a result of collisions. – Particles must collide in the correct orientation – Collision must be energetic enough to disrupt bonds

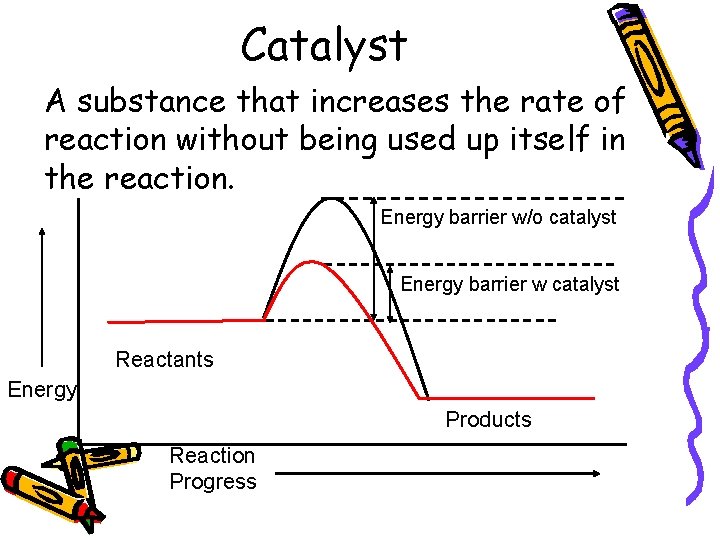
Catalyst A substance that increases the rate of reaction without being used up itself in the reaction. Energy barrier w/o catalyst Energy barrier w catalyst Reactants Energy Products Reaction Progress


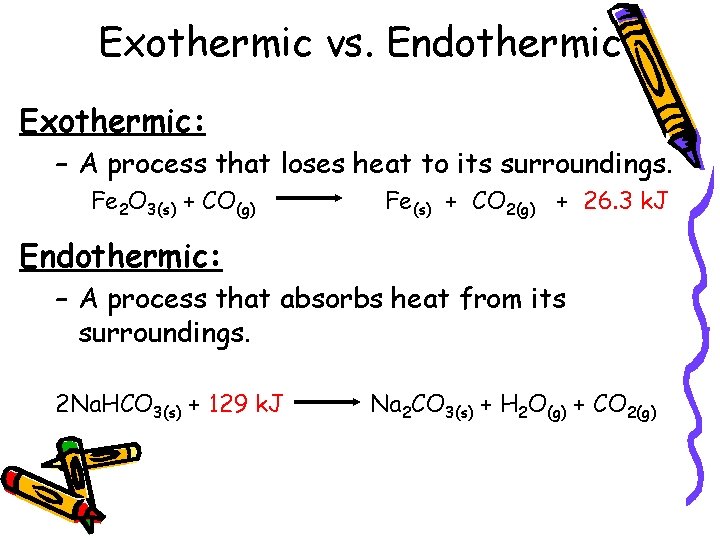
Exothermic vs. Endothermic Exothermic: – A process that loses heat to its surroundings. Fe 2 O 3(s) + CO(g) Fe(s) + CO 2(g) + 26. 3 k. J Endothermic: – A process that absorbs heat from its surroundings. 2 Na. HCO 3(s) + 129 k. J Na 2 CO 3(s) + H 2 O(g) + CO 2(g)

http: //www. mhhe. com/physsci/chemistry/essentialchemistry/flash/activa 2. swf

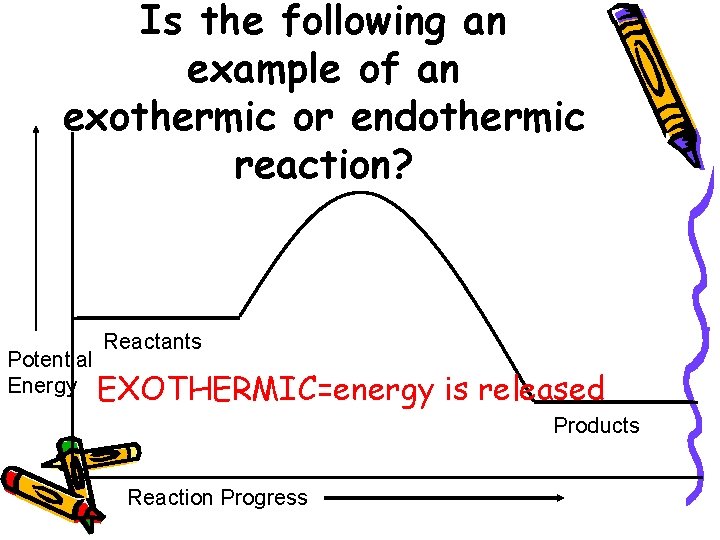
Is the following an example of an exothermic or endothermic reaction? Reactants Potential Energy EXOTHERMIC=energy is released Products Reaction Progress

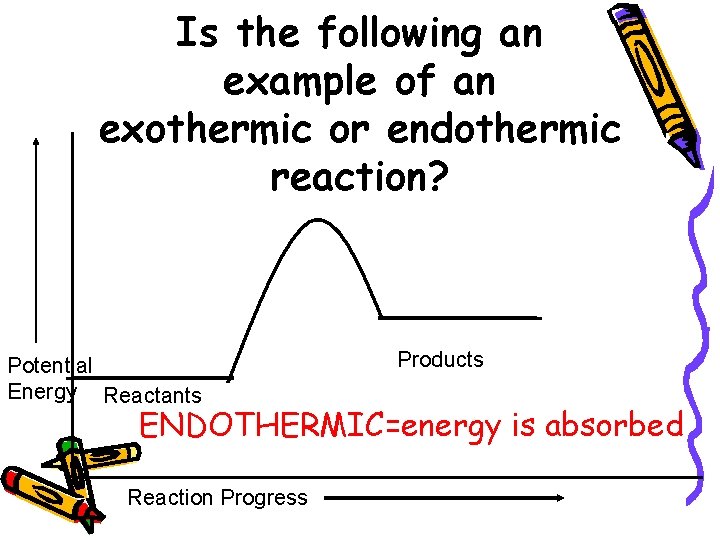
Is the following an example of an exothermic or endothermic reaction? Potential Energy Reactants Products ENDOTHERMIC=energy is absorbed Reaction Progress

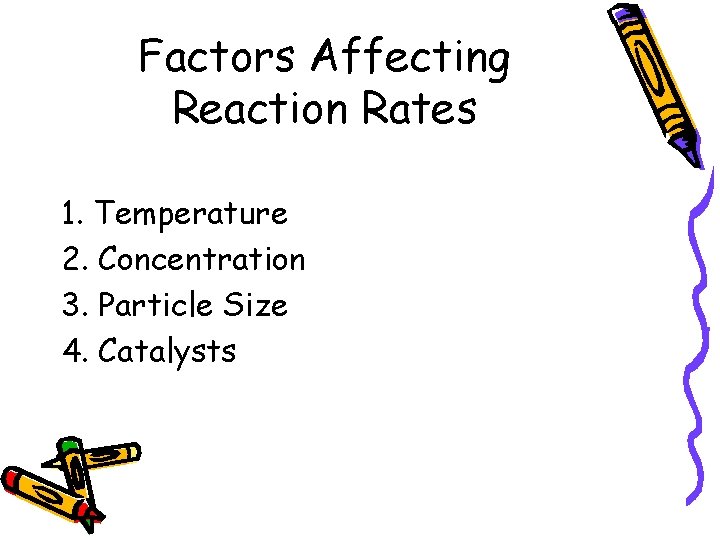
Factors Affecting Reaction Rates 1. Temperature 2. Concentration 3. Particle Size 4. Catalysts

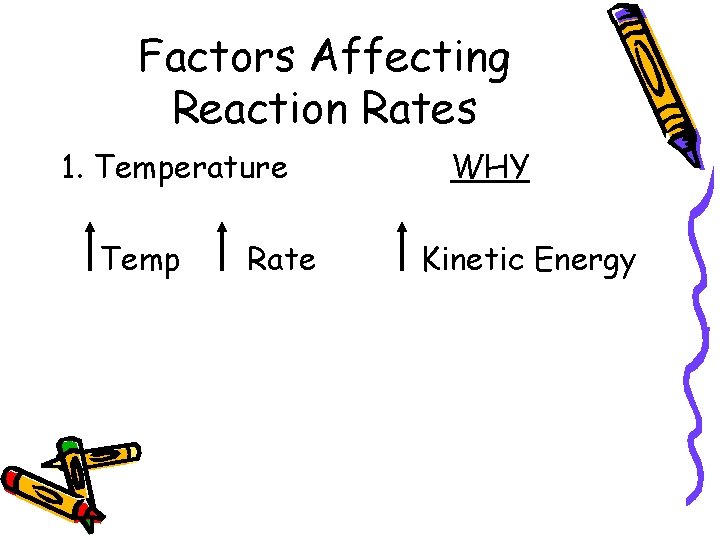
Factors Affecting Reaction Rates 1. Temperature Temp Rate WHY Kinetic Energy

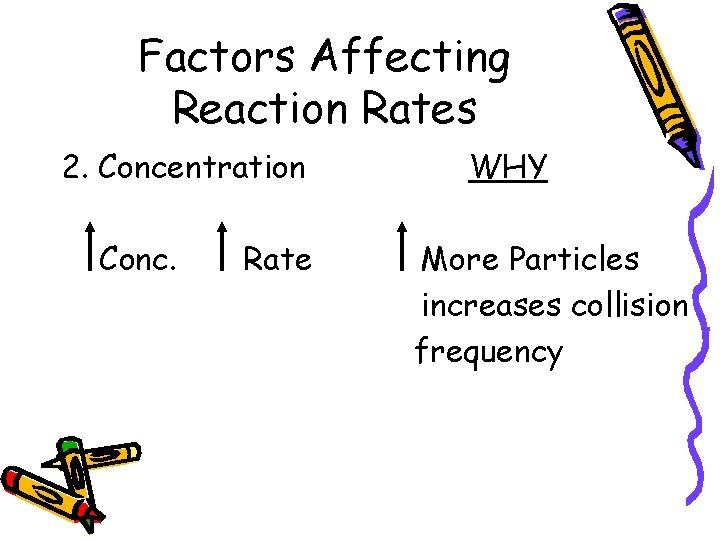
Factors Affecting Reaction Rates 2. Concentration Conc. Rate WHY More Particles increases collision frequency

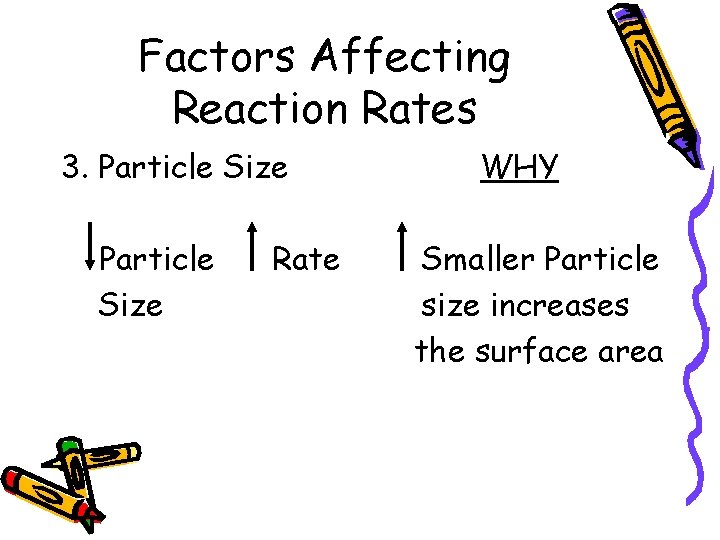
Factors Affecting Reaction Rates 3. Particle Size Rate WHY Smaller Particle size increases the surface area

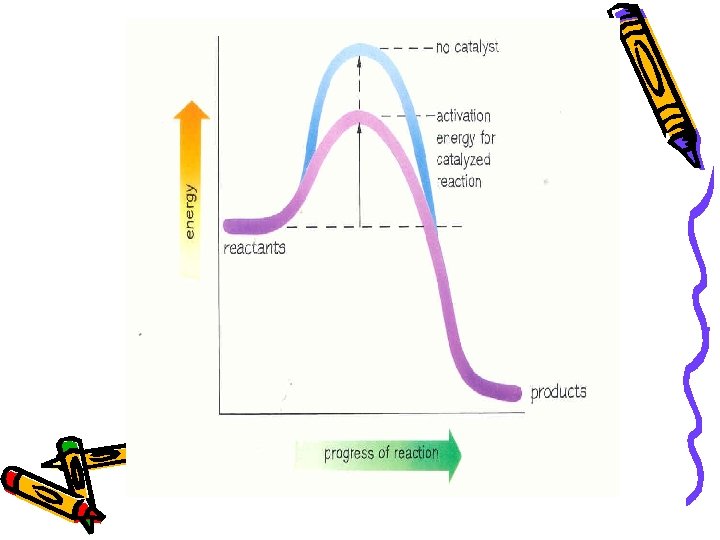
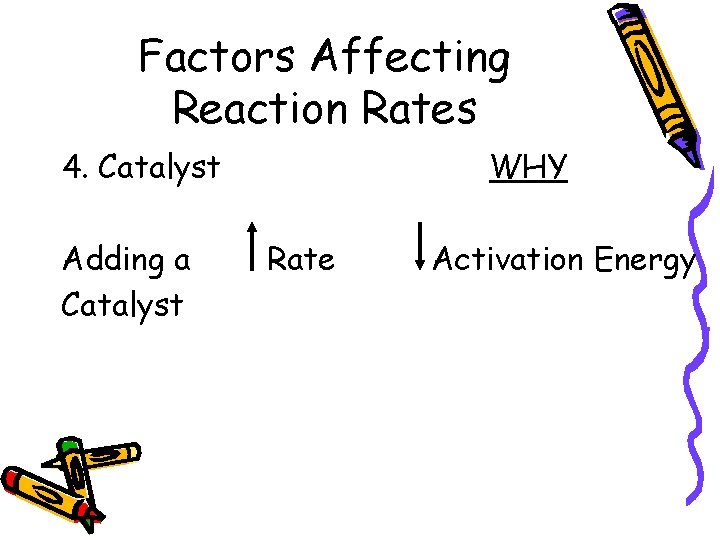
Factors Affecting Reaction Rates 4. Catalyst Adding a Catalyst WHY Rate Activation Energy

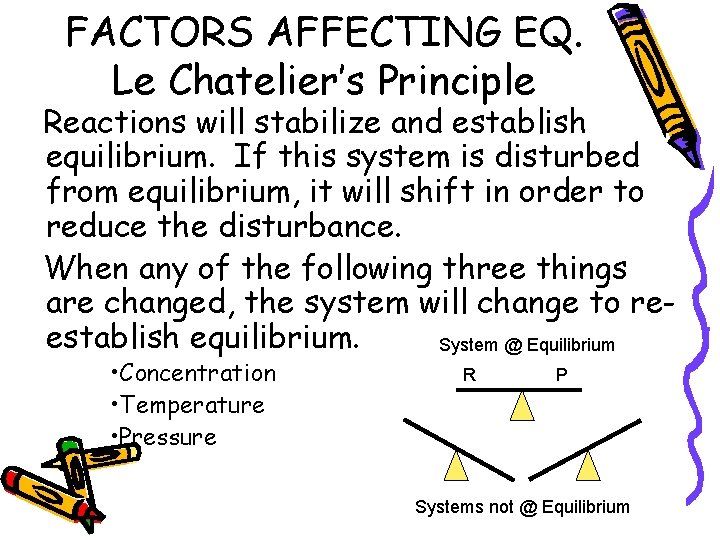
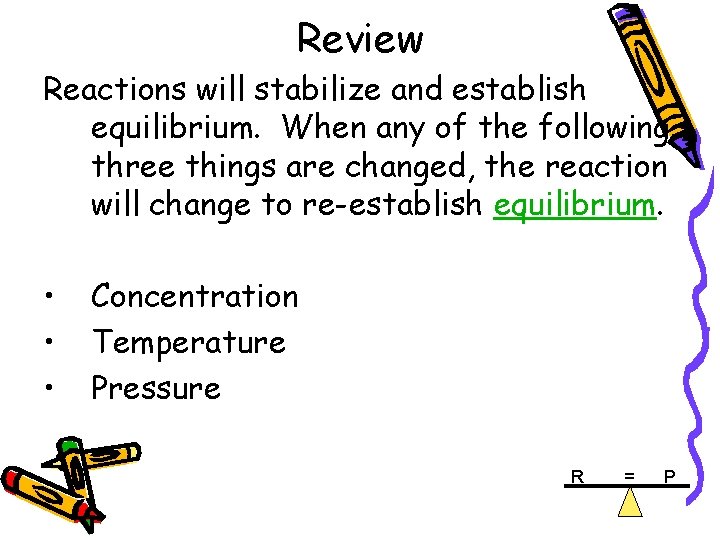
FACTORS AFFECTING EQ. Le Chatelier’s Principle Reactions will stabilize and establish equilibrium. If this system is disturbed from equilibrium, it will shift in order to reduce the disturbance. When any of the following three things are changed, the system will change to reestablish equilibrium. System @ Equilibrium • Concentration • Temperature • Pressure R P Systems not @ Equilibrium

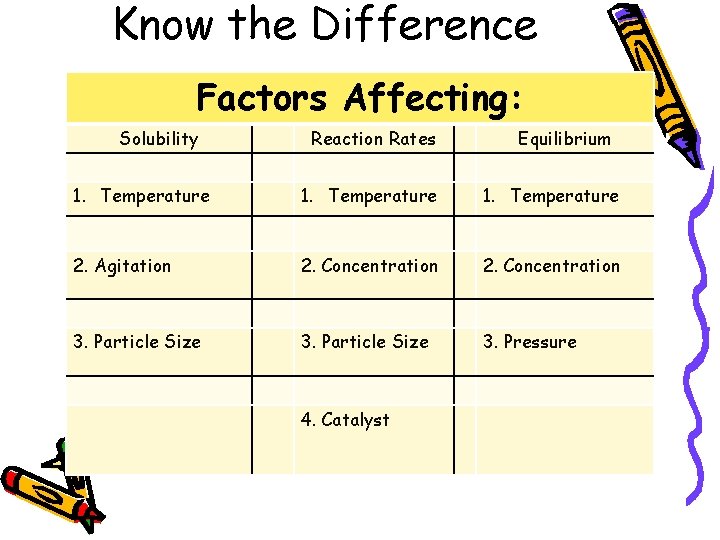
Know the Difference Factors Affecting: Solubility Reaction Rates Equilibrium 1. Temperature 2. Agitation 2. Concentration 3. Particle Size 3. Pressure 4. Catalyst

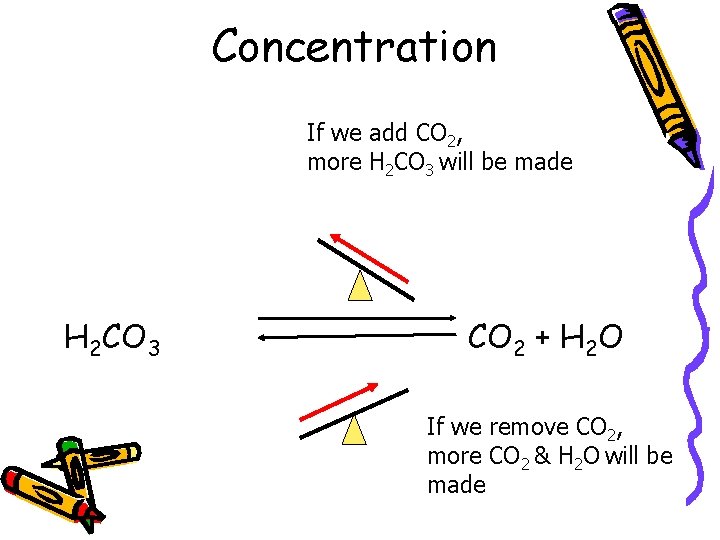
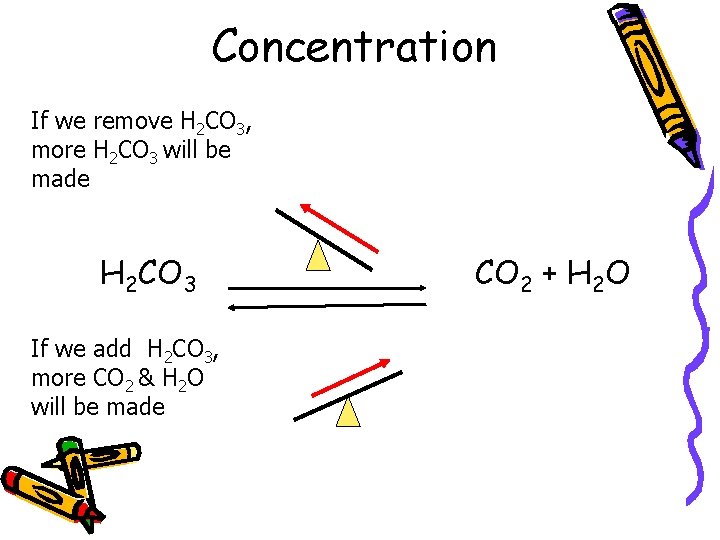
Concentration If we add CO 2, more H 2 CO 3 will be made H 2 CO 3 CO 2 + H 2 O If we remove CO 2, more CO 2 & H 2 O will be made

Concentration If we remove H 2 CO 3, more H 2 CO 3 will be made H 2 CO 3 If we add H 2 CO 3, more CO 2 & H 2 O will be made CO 2 + H 2 O

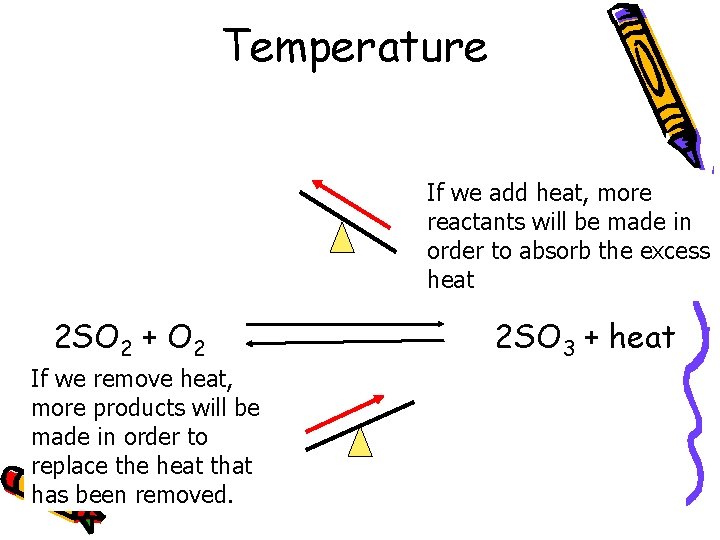
Temperature If we add heat, more reactants will be made in order to absorb the excess heat 2 SO 2 + O 2 If we remove heat, more products will be made in order to replace the heat that has been removed. 2 SO 3 + heat

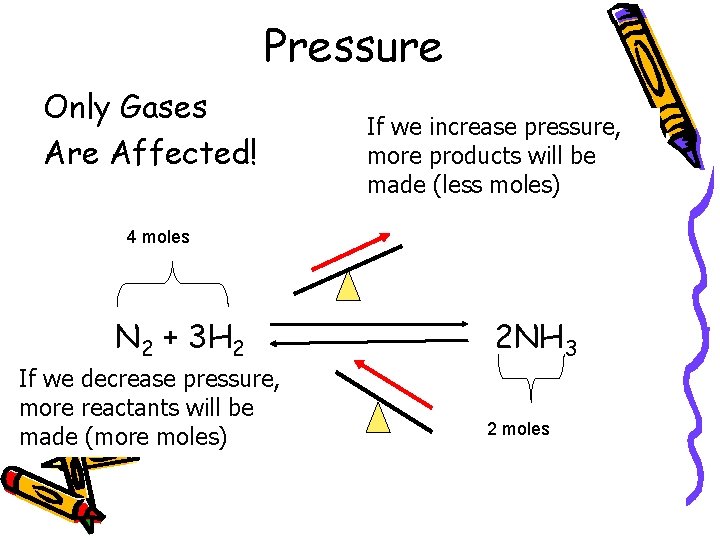
Pressure Only Gases Are Affected! If we increase pressure, more products will be made (less moles) 4 moles N 2 + 3 H 2 If we decrease pressure, more reactants will be made (more moles) 2 NH 3 2 moles

Review Reactions will stabilize and establish equilibrium. When any of the following three things are changed, the reaction will change to re-establish equilibrium. • • • Concentration Temperature Pressure R = P