Enzymes grouped in 6 major classes p 643


- Slides: 20

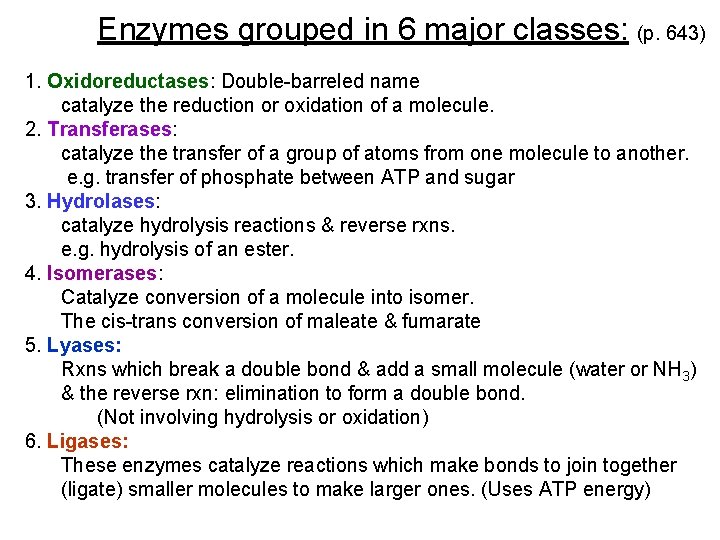
Enzymes grouped in 6 major classes: (p. 643) 1. Oxidoreductases: Double-barreled name catalyze the reduction or oxidation of a molecule. 2. Transferases: catalyze the transfer of a group of atoms from one molecule to another. e. g. transfer of phosphate between ATP and sugar 3. Hydrolases: catalyze hydrolysis reactions & reverse rxns. e. g. hydrolysis of an ester. 4. Isomerases: Catalyze conversion of a molecule into isomer. The cis-trans conversion of maleate & fumarate 5. Lyases: Rxns which break a double bond & add a small molecule (water or NH 3) & the reverse rxn: elimination to form a double bond. (Not involving hydrolysis or oxidation) 6. Ligases: These enzymes catalyze reactions which make bonds to join together (ligate) smaller molecules to make larger ones. (Uses ATP energy)

Each class has more specific subclasses: 1. Oxidoreductases: oxidases and reductases dehydrogenases (remove 2 H; make double bond) 2. Transferases: transaminases (amino group), kinases (phosphate group) 3. Hydrolases: lipases, proteases, nucleases, carbohydrases, and phosphatases (break ester and amide bonds) 4. Isomerases: racemases (D to L or L to D forms) mutases (structural isomer D) 5. Lyases: dehydratases (remove H 2 O from substrate) decarboxylases (remove CO 2 from substrate) deaminases (remove NH 3 from substrate) hydratases (add H 2 O to substrate) 6. Ligases: synthetases (hook 2 substrates together) carboxylases (add CO 2 from substrate)

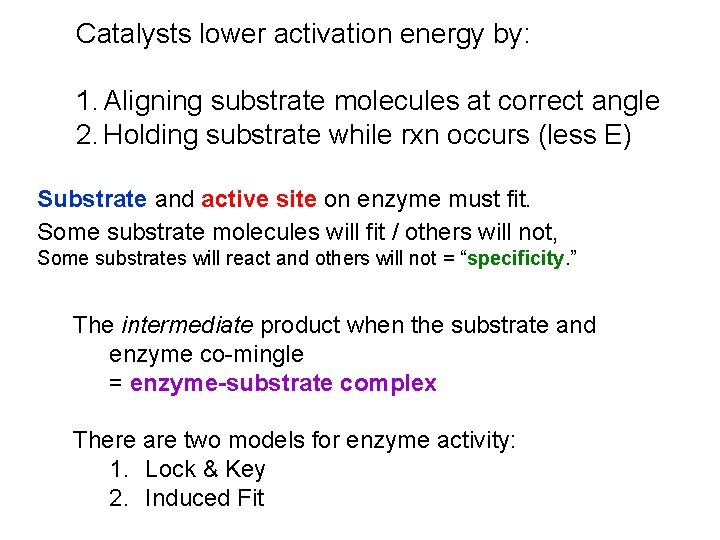
Catalysts lower activation energy by: 1. Aligning substrate molecules at correct angle 2. Holding substrate while rxn occurs (less E) Substrate and active site on enzyme must fit. Some substrate molecules will fit / others will not, Some substrates will react and others will not = “specificity. ” The intermediate product when the substrate and enzyme co-mingle = enzyme-substrate complex There are two models for enzyme activity: 1. Lock & Key 2. Induced Fit

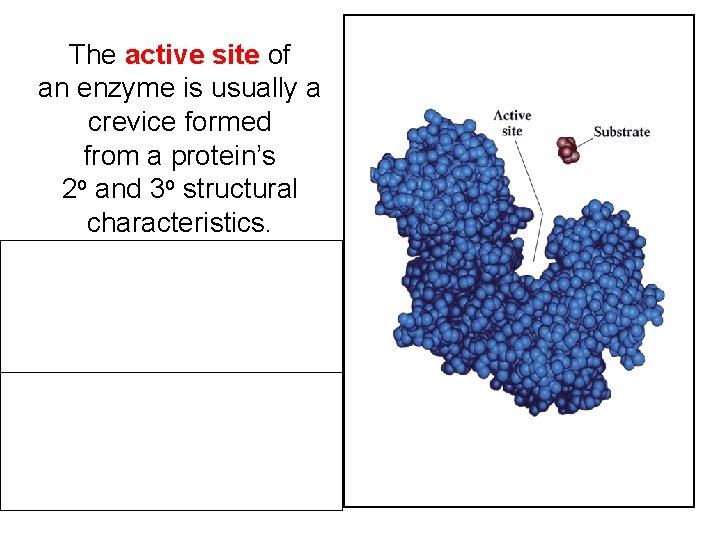
The active site of an enzyme is usually a crevice formed from a protein’s 2 o and 3 o structural characteristics. Usually is only a small portion of protein structure. May be multiple active sites

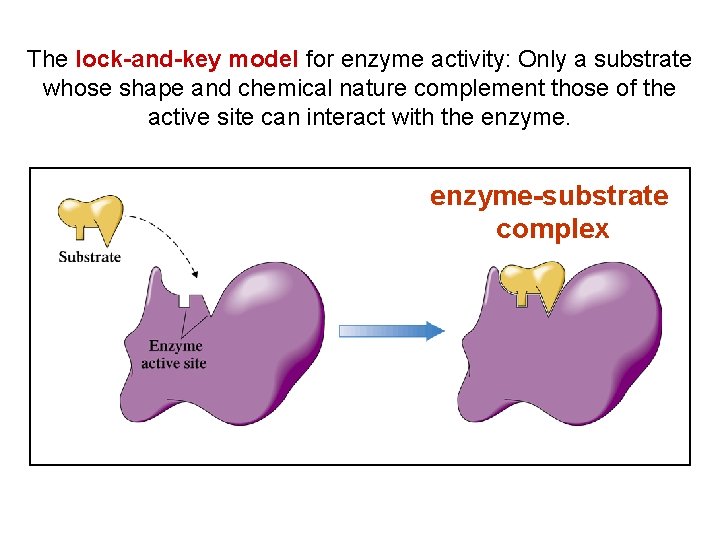
The lock-and-key model for enzyme activity: Only a substrate whose shape and chemical nature complement those of the active site can interact with the enzyme-substrate complex

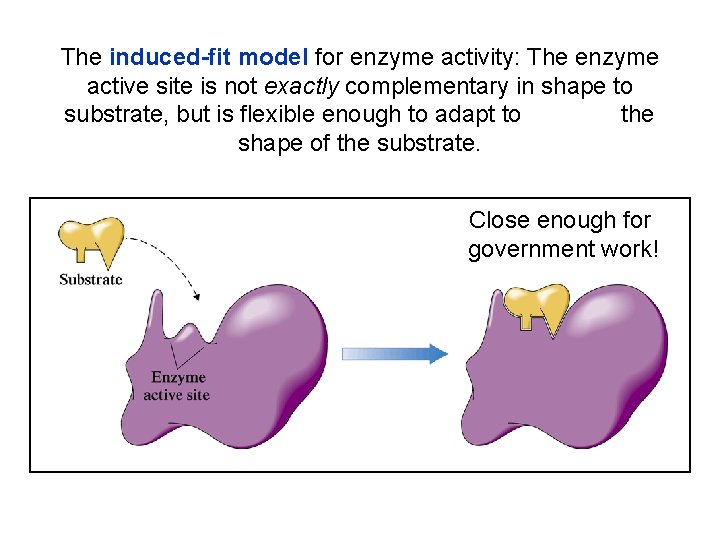
The induced-fit model for enzyme activity: The enzyme active site is not exactly complementary in shape to substrate, but is flexible enough to adapt to the shape of the substrate. Close enough for government work!

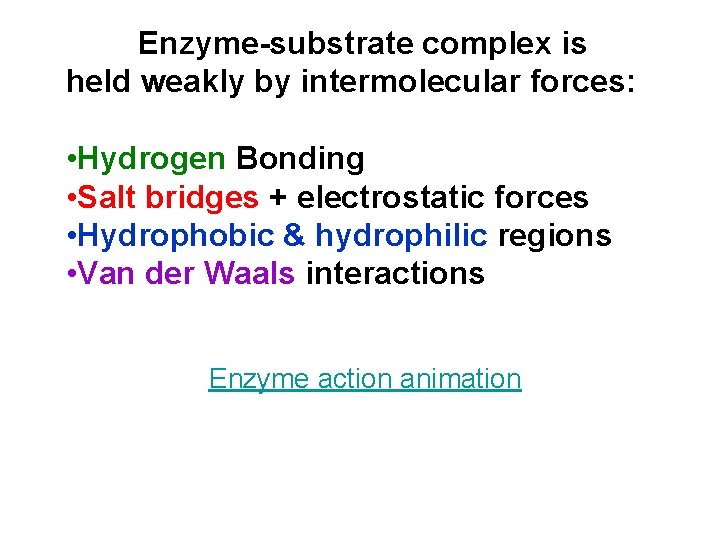
Enzyme-substrate complex is held weakly by intermolecular forces: • Hydrogen Bonding • Salt bridges + electrostatic forces • Hydrophobic & hydrophilic regions • Van der Waals interactions Enzyme action animation

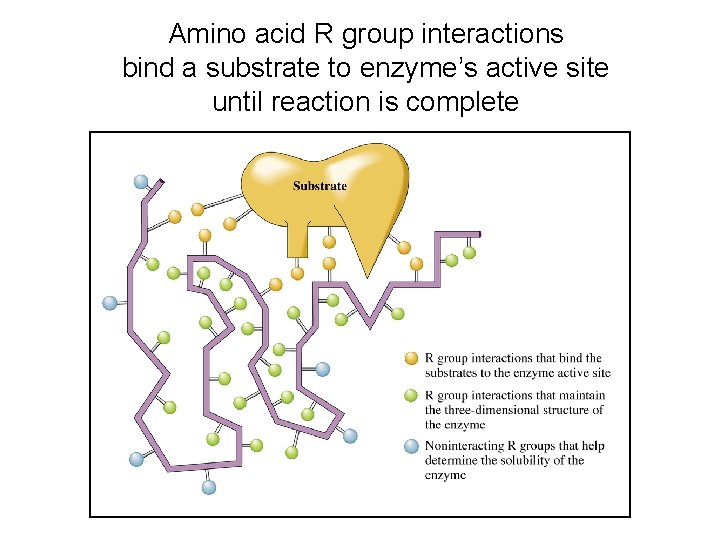
Amino acid R group interactions bind a substrate to enzyme’s active site until reaction is complete

Enzyme specificity levels Enzymes can vary in their specificity: Some active sites are very shape specific and bind to only one compound. Others are more generalized and focus on a similar structure found on various “related” molecules. 1. Absolute specificity 2. Stereochemical specificity 3. Group specificity 4. Linkage specificity

Factors affecting Enzyme Activity Four main factors affect the reaction rate: • Temperature changes • p. H changes • Changes in substrate concentration • Changes in enzyme concentration

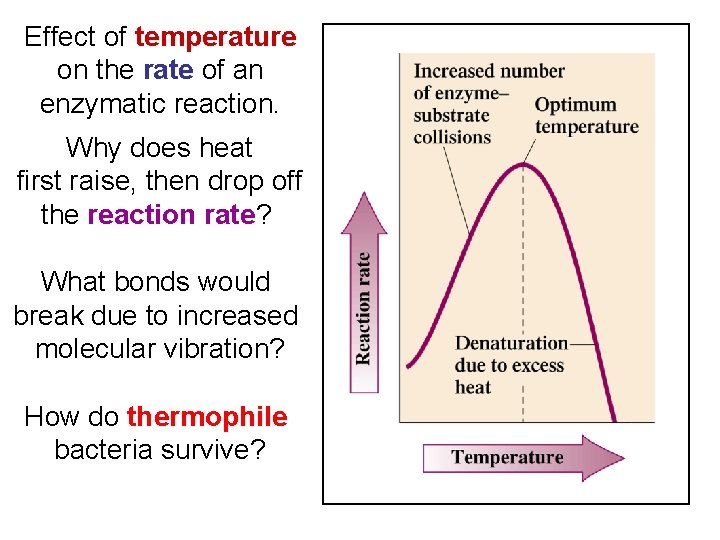
Effect of temperature on the rate of an enzymatic reaction. Why does heat first raise, then drop off the reaction rate? What bonds would break due to increased molecular vibration? How do thermophile bacteria survive?

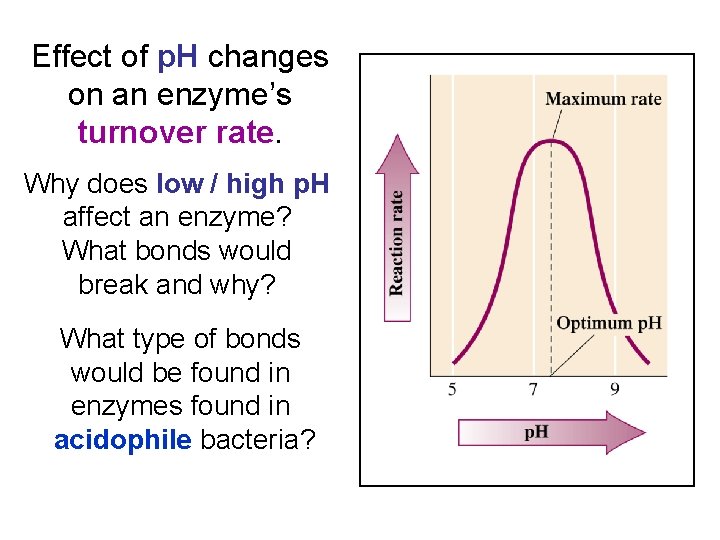
Effect of p. H changes on an enzyme’s turnover rate. Why does low / high p. H affect an enzyme? What bonds would break and why? What type of bonds would be found in enzymes found in acidophile bacteria?

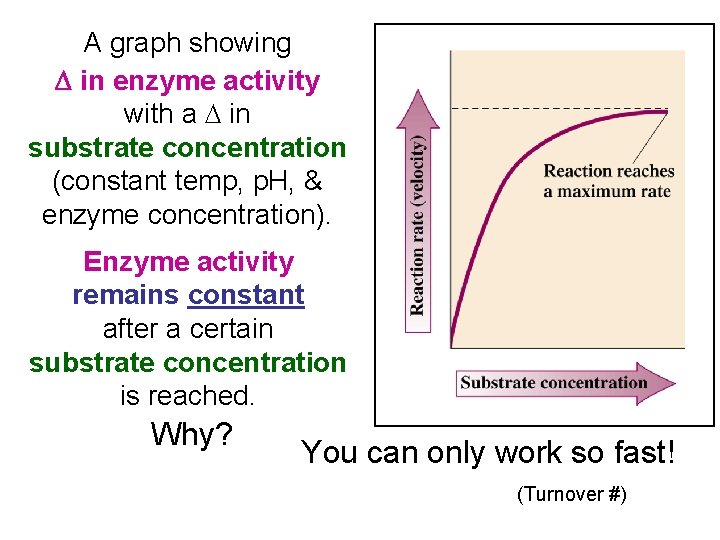
A graph showing D in enzyme activity with a D in substrate concentration (constant temp, p. H, & enzyme concentration). Enzyme activity remains constant after a certain substrate concentration is reached. Why? You can only work so fast! (Turnover #)

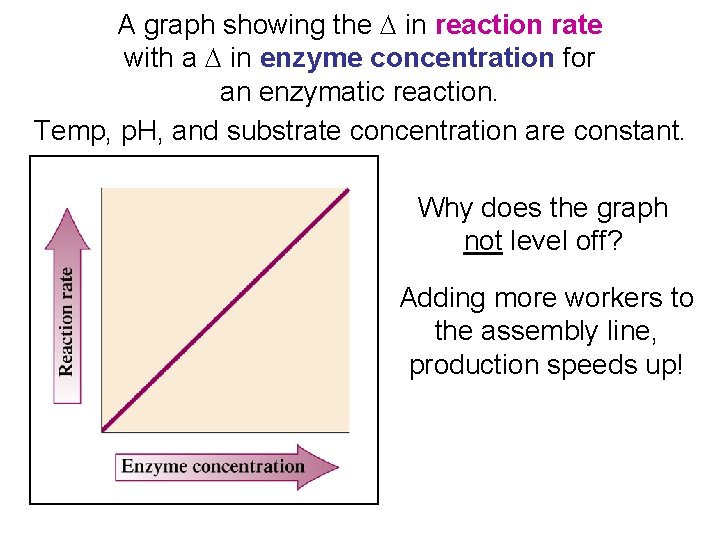
A graph showing the D in reaction rate with a D in enzyme concentration for an enzymatic reaction. Temp, p. H, and substrate concentration are constant. Why does the graph not level off? Adding more workers to the assembly line, production speeds up!

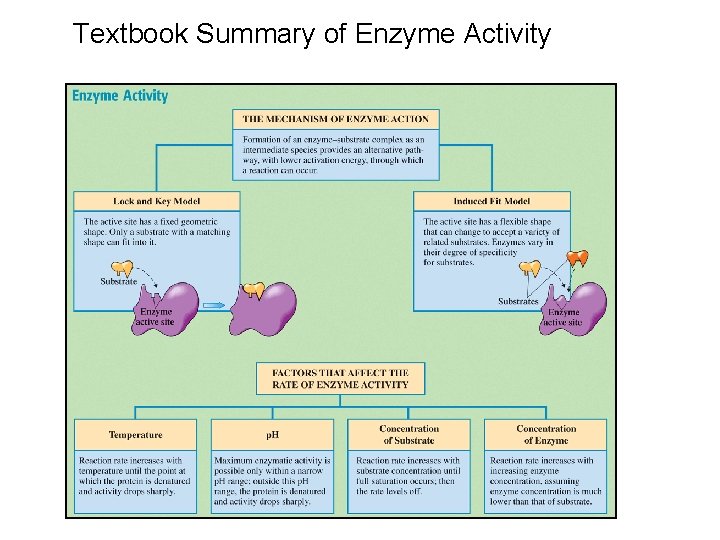
Textbook Summary of Enzyme Activity

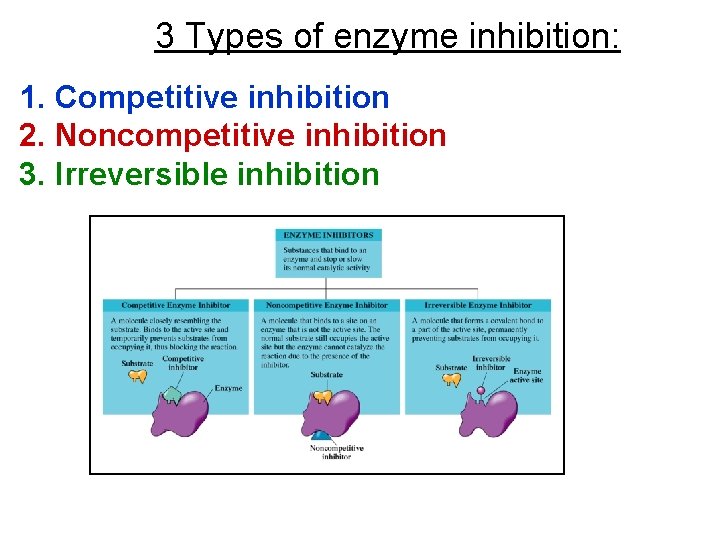
3 Types of enzyme inhibition: 1. Competitive inhibition 2. Noncompetitive inhibition 3. Irreversible inhibition

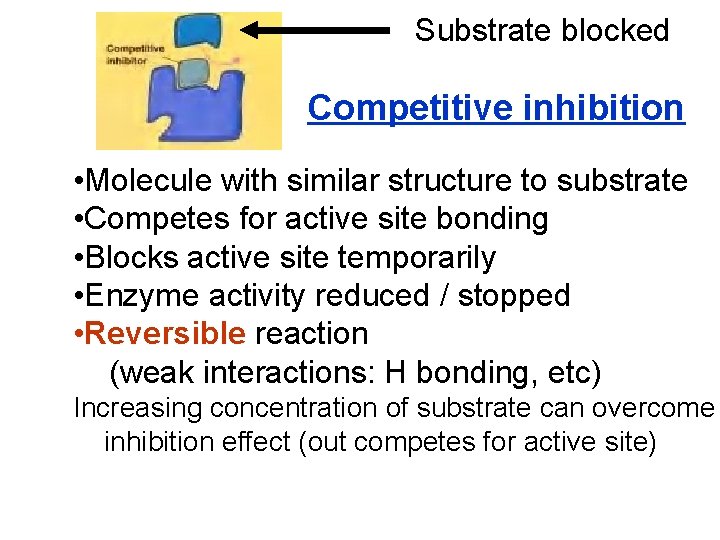
Substrate blocked Competitive inhibition • Molecule with similar structure to substrate • Competes for active site bonding • Blocks active site temporarily • Enzyme activity reduced / stopped • Reversible reaction (weak interactions: H bonding, etc) Increasing concentration of substrate can overcome inhibition effect (out competes for active site)

e. g. antihistamines vs histadine decarboxylation (which converts histadine to histamine) histamine Viagra: interferes with nitric oxide breakdown (NO is a vasodilator)

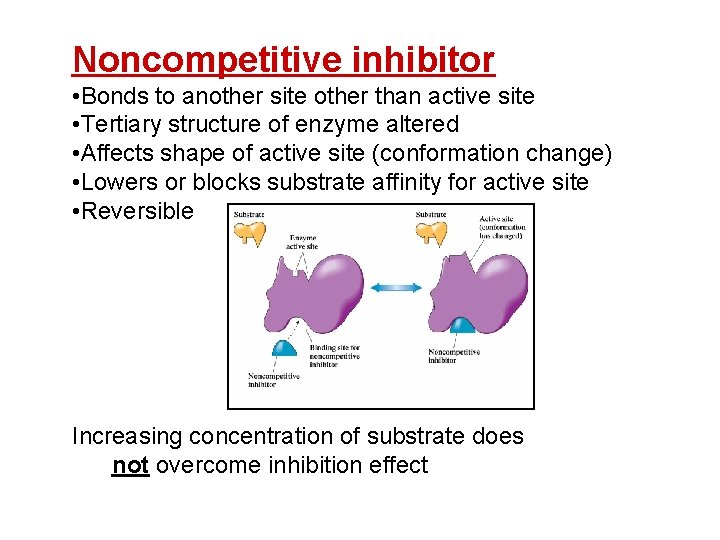
Noncompetitive inhibitor • Bonds to another site other than active site • Tertiary structure of enzyme altered • Affects shape of active site (conformation change) • Lowers or blocks substrate affinity for active site • Reversible Increasing concentration of substrate does not overcome inhibition effect

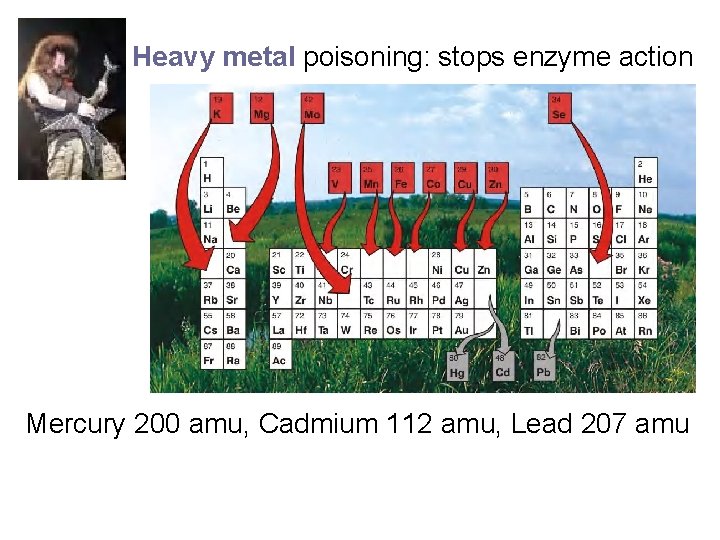
Heavy metal poisoning: stops enzyme action Mercury 200 amu, Cadmium 112 amu, Lead 207 amu