Chapter 2 MINERALOGY A naturally occurring inorganic solid








- Slides: 16

Chapter 2: MINERALOGY A naturally occurring, inorganic solid with a characteristic chemical composition and a crystalline structure. 1. Naturally occurring: Synthetic diamond, concrete, and glass etc. don’t count as minerals. 2. Inorganic: Coal and oil eliminated. 3. Chemical Composition of minerals: Elemental composition and type of bonding determine the type of mineral and the mineral properties. 4. Crystalline Nature – Characteristic of Internal Structure Atomic structure expressed in external crystalline form and in other physical properties. Crystalline – having crystal form – orderly array of atoms Crystal – any substance, whose atoms are arranged on a regular, periodically repeated pattern.

Physical Properties of minerals Different minerals can be uniquely identified by different properties. • Crystal habit – the characteristic shape of a mineral and the manner in which aggregates of crystals grow. • Cleavage – the tendency of some minerals to break along flat surfaces, e. g. halite has cubic cleavage, and calcite exhibits rhombohedral cleavage. • Hardness – the resistance of a mineral to scratching. Mohs scale, Talc =1, diamond = 10, finger nail slightly > 2, and knife blade slightly > 5.

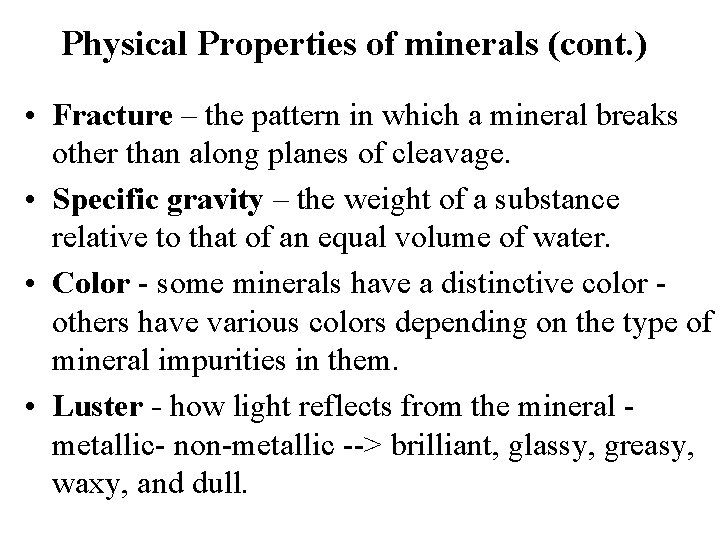
Physical Properties of minerals (cont. ) • Fracture – the pattern in which a mineral breaks other than along planes of cleavage. • Specific gravity – the weight of a substance relative to that of an equal volume of water. • Color - some minerals have a distinctive color others have various colors depending on the type of mineral impurities in them. • Luster - how light reflects from the mineral metallic- non-metallic --> brilliant, glassy, greasy, waxy, and dull.

Physical Properties of minerals (cont. ) • Miscellaneous - specific properties - graphite writes on paper - talc feels soapy (soapstone) - magnetite is magnetic - calcite has double reflections - calcite reacts with 10% hydrochloric acid - striation – fine parallel lines that appear to be scribed on to cleavage faces of the feldspar (plagioclse) - tenacity – the resistance a mineral shows to various destructive mechanism.

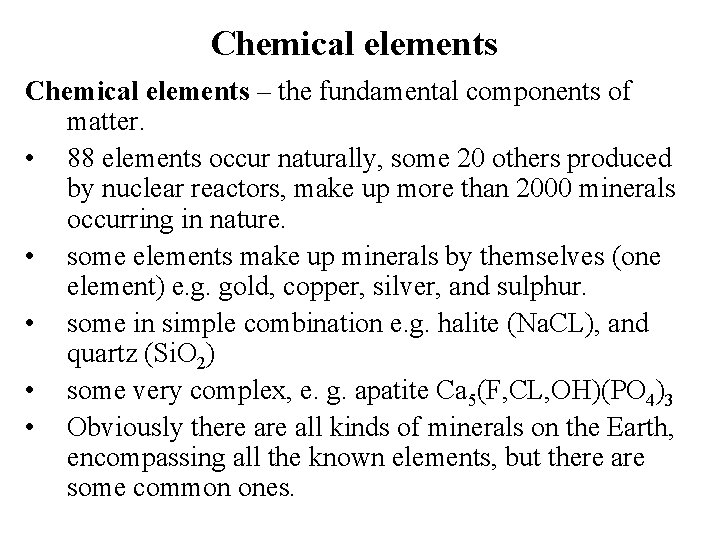
Chemical elements – the fundamental components of matter. • 88 elements occur naturally, some 20 others produced by nuclear reactors, make up more than 2000 minerals occurring in nature. • some elements make up minerals by themselves (one element) e. g. gold, copper, silver, and sulphur. • some in simple combination e. g. halite (Na. CL), and quartz (Si. O 2) • some very complex, e. g. apatite Ca 5(F, CL, OH)(PO 4)3 • Obviously there all kinds of minerals on the Earth, encompassing all the known elements, but there are some common ones.

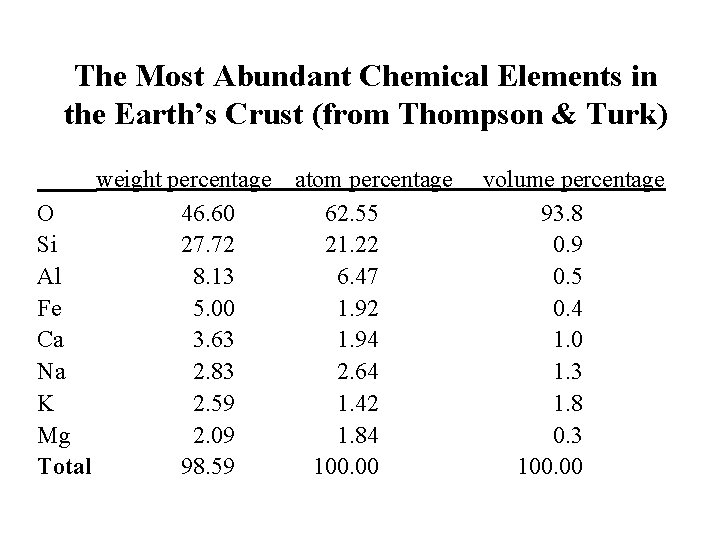
The Most Abundant Chemical Elements in the Earth’s Crust (from Thompson & Turk) weight percentage O 46. 60 Si 27. 72 Al 8. 13 Fe 5. 00 Ca 3. 63 Na 2. 83 K 2. 59 Mg 2. 09 Total 98. 59 atom percentage 62. 55 21. 22 6. 47 1. 92 1. 94 2. 64 1. 42 1. 84 100. 00 volume percentage 93. 8 0. 9 0. 5 0. 4 1. 0 1. 3 1. 8 0. 3 100. 00

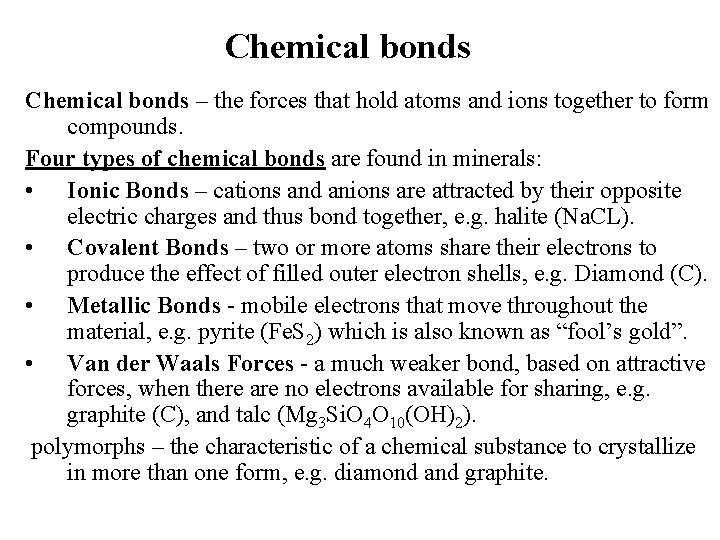
Chemical bonds – the forces that hold atoms and ions together to form compounds. Four types of chemical bonds are found in minerals: • Ionic Bonds – cations and anions are attracted by their opposite electric charges and thus bond together, e. g. halite (Na. CL). • Covalent Bonds – two or more atoms share their electrons to produce the effect of filled outer electron shells, e. g. Diamond (C). • Metallic Bonds - mobile electrons that move throughout the material, e. g. pyrite (Fe. S 2) which is also known as “fool’s gold”. • Van der Waals Forces - a much weaker bond, based on attractive forces, when there are no electrons available for sharing, e. g. graphite (C), and talc (Mg 3 Si. O 4 O 10(OH)2). polymorphs – the characteristic of a chemical substance to crystallize in more than one form, e. g. diamond and graphite.

Mineral Categories 1) Rock-forming minerals Most common minerals in the Earth’s crust, e. g. olivine, pyroxene, amphibole, mica, the clay minerals, feldspar, quartz, calcite and dolomite. 2) Accessory minerals Minerals that are common but usually are found only in small amounts, e. g. chlorite, garnet, hematite, limonite, magnetite, and pyrite. 3) Gems A mineral that is prized primarily for its beauty. (Although some gems, like diamonds are also used industrially), e. g. diamond, emerald, ruby, and sapphire.

Mineral Categories (cont. ) 4) Ore minerals Minerals from which metals or other elements can be profitably recovered, e. g. native gold, native silver, chalcopyrite, galena, and sphalerite. 5) Industrial minerals Minerals are industrially important, but are mined for purposes other than the extraction of metals, e. g. halite for table salt.

Mineral Classification (according to their anions) 1) Native elements: e. g. gold, silver, platinum, and copper. 2) Oxides: Elements plus oxygen, Simple formulas, e. g. ice H 2 O, Hematite Fe 2 O 3, Magnetite Fe 3 O 4, quartz Si. O 2, corundum Al 2 O 3, etc. Metal ores – iron, aluminum, and tin. 3) Sulfides: Elements plus sulfur, e. g. Galena (Pb. S), Pyrite (Fe. S 2), sphalerite (Zn. S), and Chalcopyrite (Cu. Fe. S 2. ). Mineral ores – lead, zinc, and copper. 4) Sulfates: Elements plus (SO 4)2 -, e. g. Gypsum (Ca. SO 4 2 H 2 O), anhydrite (Ca. SO 4), and barite (Ba. SO 4).

Mineral Classification (according to their anions) (cont. ) 5) Halides: Halogen elements plus various cations, e. g. Halite (Na. Cl), and sylvite (KCL). Table salt, and fertilizer 6) Phosphates: Elements plus (PO 4)3 -, e. g. apatite, and (Ca 5(F, Cl, OH)(PO 4)3. ). fertilizer 7) Carbonates: Elements plus (CO 3)2 - , e. g. calcite (Ca. CO 3), and Dolomite (Ca. Mg(CO 3)2). cement

Mineral Classification (according to their anions) (cont. ) 8) Silicates: elements plus silica ion(Si. O 4)4 -, silicates make up 95% of the Earth’s crust. basic building block – silica tetrahedron - complex ion w/ negative charge of 4, 1 Si 4+, and 4 O 2 - = 8 -. Oxygens stay as close as possible, so it’s very stable. • Single tetrahedron, e. g. olivine. • Ring – six tetrahedron, each sharing two oxygen, e. g beryl. • Single chain – each shares two oxygens, e. g. augite. • Double chain – various sharing. outward pointing sharing 2 oxygens. Inward pointing sharing 3 oxygens, e. g. hornblendes. • Sheets – each shares 3 oxygen, e. g, Mica, clay minerals. • 3 - D (framework) – all four oxygen atoms shared, e. g. quartz, and potassium feldspar.

CLAY MINERALOGY • Clays are composed of very fine particles < 0. 002 mm diameter particles (crystals if you like). • Clays are a serious concern in engineering geology. • Many clays can be very strong (bearing capacity) under the right conditions. • All clays can be very weak if disturbed or at too high a water content. • Types Kaolinite - illite, vermiculite - smectite chlorite

Building blocks of clay minerals • Silica tetrahedral sheet – Cation: Si 4+ – Anion: O 2 - • Octahedral (gibbsite) sheet – Cation Al 3+ – Anion: OH- (hydroxyl) ---------------------------- Atmos (neutral) – ions (lost or gained electrons) • cation (lost, positive) • anion (gained, negative)

Clay mineral classification • Two layer clay minerals – e. g. kaolinite: • 2 -unit clay • Relatively stable – due to the hydrogen bond • Low activity • Three layer clay minerals – e. g. illite • • 3 -unit clay Oxygen – potassium – oxygen bond Not a weak bond but not as strong as hydrogen bond Rich in potassium

Clay mineral classification (cont. ) • Three layer clay minerals – e. g. montmorillonite • • • 3 -unit clay Bonded by n. H 2 O and exchangeable cations Almost no substitute of Al 3+ for Si 4+ Charge deficiency doe to lack of Al 3+ Hydrated ions are attracted weekly by a force from the center of the sheet