Chapter 1 The Study of Change Dr Nouf













- Slides: 15

Chapter 1: The Study of Change Dr. Nouf Alotaibi

Ø Which of the following would NOT be a physical change? a. freezing water to make ice cubes. b. melting gold to make jewelry. c. burning a piece of paper. d. tearing a piece of aluminum foil. Ø Which of the following would be a chemical change? a. perfume evaporating on your skin. b. melting butter for popcorn. c. burning sugar. d. a hot glass breaking when placed in cold water.

Ø Which of the following is an example of a physical property? A) Combustibility B) Corrosiveness C) Explosiveness D) Density Ø Which of the following would NOT be an extensive property? A. volume of a substance in beaker. B. mass of a soccer ball. C. boiling point of water. D. length of a class room.

Ø What is the SI unit of mass? A) Meter B) Centimeter C) Gram D) Kilogram (kg) Ø The symbol for density in SI system is: A. m 3/s B. kg C. kg/m 3 D. g/ml

Ø Convert 240 K and 468 K to the Celsius scale. A) 513 o. C and 741 o. C B) -59 o. C and 351 o. C C) -18. 3 o. C and 108 o. C D) -33 o. C and 295 o. C

Ø Calculate the volume occupied by 4. 50 X 102 g of gold (density = 19. 3 g/cm 3). A) 23. 3 cm 3 B) 8. 69 x 103 cm 3 C) 19. 3 cm 3 D) 450 cm 3 Ø The melting point of bromine is -7 o. C. What is this melting point expressed in o. F? A) 45 o. F B) -28 o. F C) -13 o. F D) 19 o. F

Ø The number of significant figures in 0. 053 is A. 3 B. 1 C. 4 D. 2 Ø What is the Scientific form for 3, 500, 000 A. 3. 5 x 109 B. 3 x 109 C. 3. 5 x 108 D. 3. 50 x 109

Ø How many significant figures are there in the measurement 3. 4080 g? A) 6 B) 5 C) 4 D) 3 E) 2 Ø Multiply (4 x 105) (6. 2 x 107) 2. 48 x 1013

Ø Convert 357, 096 to Scientific Notation 3. 57096 x 105 Ø Convert 0. 005600 to Scientific Notation 5. 600 x 10 -3

• Given: 2. 46 x 106 + 3. 476 x 103 Ø Shift decimal 3 places to the left for 103. Ø Move: 0. 003476 x 103+3 Ø Add: 2. 46 x 106 +. 003476 x 106 Answer: 2. 463 x 106

Given: 5. 762 x 103 – 2. 65 x 10 -1 ØShift decimal 4 places to the right for 10 -1. Ø Move: 0. 000265 x 10(-1+4) Ø Subtract: 5. 762 x 103 -. 000265 x 103 Answer: 5. 762 x 103

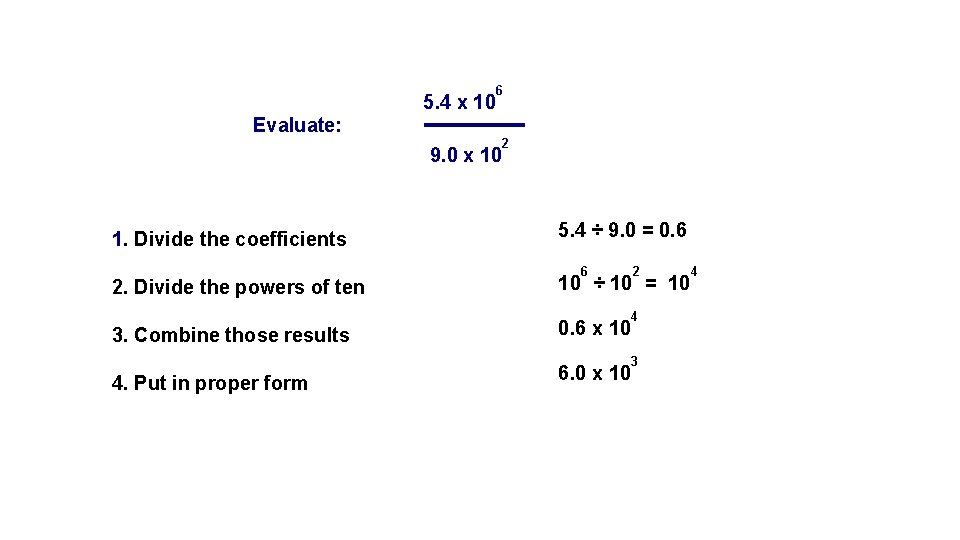
5. 4 x 10 6 Evaluate: 9. 0 x 10 1. Divide the coefficients 2 5. 4 ÷ 9. 0 = 0. 6 6 2 2. Divide the powers of ten 10 ÷ 10 = 10 3. Combine those results 0. 6 x 10 4. Put in proper form 6. 0 x 10 4 3 4

Practice Rule #1 Zeros 45. 8736 6 • All digits count . 000239 3 • Leading 0’s don’t . 00023900 5 • Trailing 0’s do 48000. 5 • 0’s count in decimal form 48000 2 • 0’s don’t count w/o decimal 3. 982 106 4 1. 00040 6 • All digits count • 0’s between digits count as well as trailing in decimal form

Addition and Subtraction. 56 __ +. 153 ___ =. 713 __. 71 82000 + 5. 32 = 82005. 32 82000 10. 0 - 9. 8742 =. 12580 . 1 10 – 9. 8742 =. 12580 0 Look for the last important digit

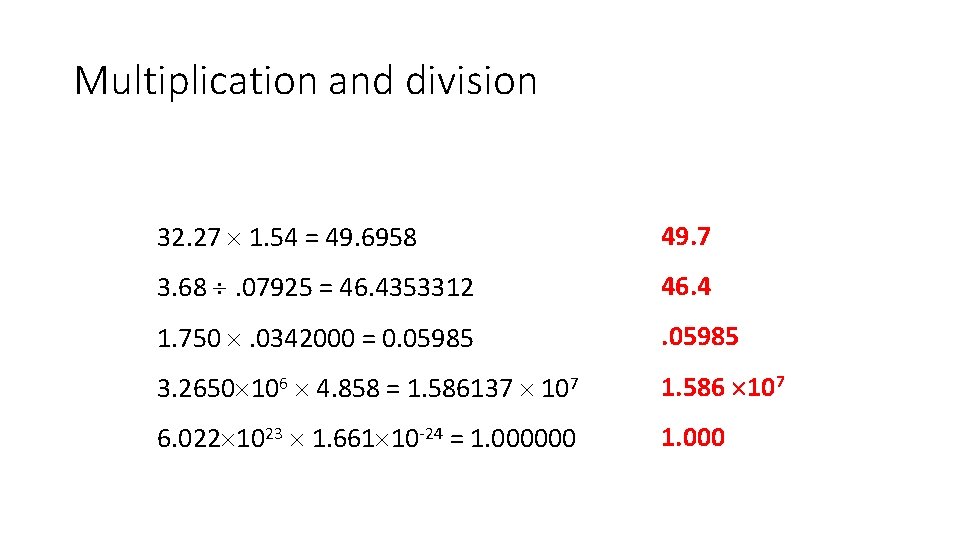
Multiplication and division 32. 27 1. 54 = 49. 6958 49. 7 3. 68 . 07925 = 46. 4353312 46. 4 1. 750 . 0342000 = 0. 05985 3. 2650 106 4. 858 = 1. 586137 107 1. 586 107 6. 022 1023 1. 661 10 -24 = 1. 000000 1. 000