Chapter 1 Introduction Why Study Physics 3 4










- Slides: 17

Chapter 1: Introduction • Why Study Physics? 3, 4 • Mathematics and Physics Speak 5, 6 • Theories and Laws • Scientific Notation and Significant Figures 16+ • Units • Dimensional Analysis • Problem Solving Techniques 29 • Approximations 30 • Graphs 32 -33 • HK: CQ: 1, 8. P: 1, 3, 5, 13, 25, 39, 49. 1

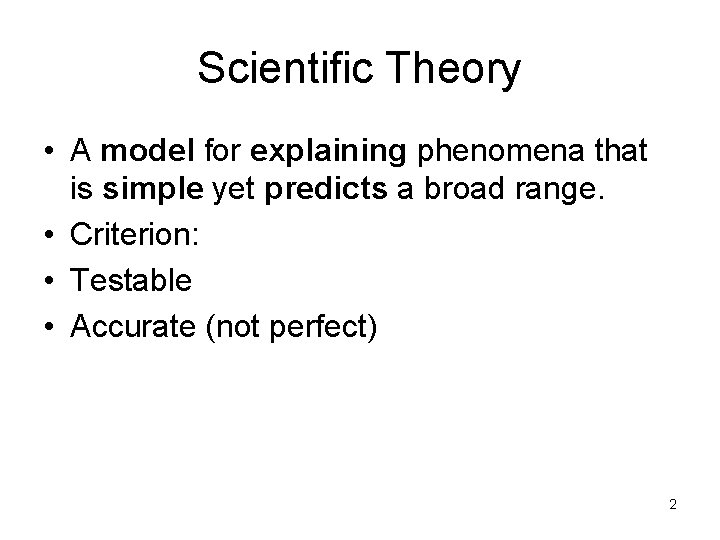
Scientific Theory • A model for explaining phenomena that is simple yet predicts a broad range. • Criterion: • Testable • Accurate (not perfect) 2

Law • A scientific law is a statement describing natural behavior which has a history of scientific replication. • Law = describing • Theory = explaining • Describing is easier than explaining! Laws usually come first. • E. g. Boyle’s Law (1662), Bernoulli’s Kinetic Theory of gases (1740). 3

SI Base Units • meter (m) • kilogram (kg) • second (s, sec) • • ampere (A) kelvin (K) mole (mol) candela (cd) 4

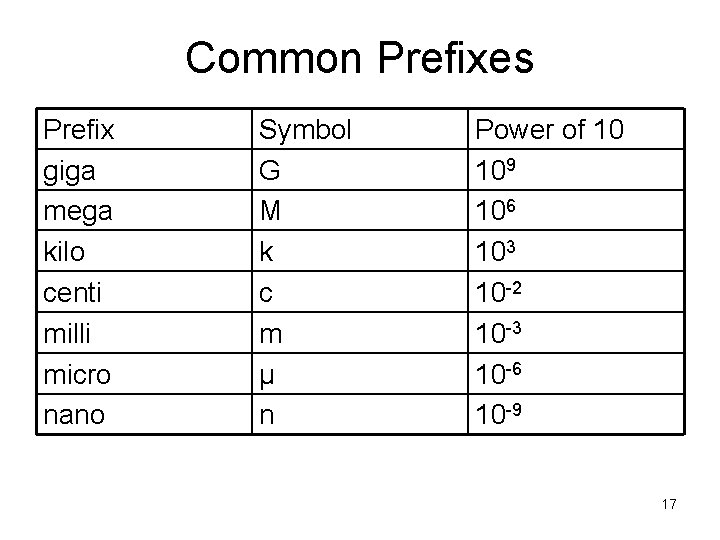
Prefixes for SI Units • • • convenient shorthand for powers of 10 examples: m = 10 -3 e. g. millimeter c = 10 -2 e. g. centimeter k = 103 e. g. kilometer 5

Other Common Units • • foot: mile: hour: / 3. 28 ft = 1 m 1 mi = 1609 m 1 h = 3600 s 6

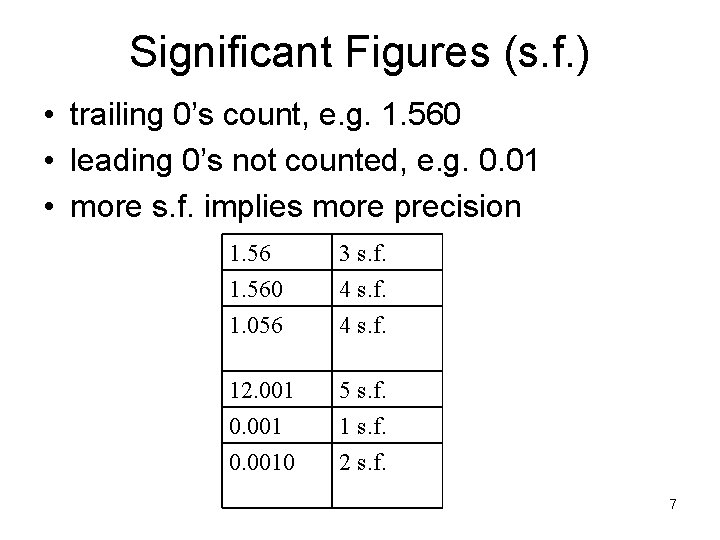
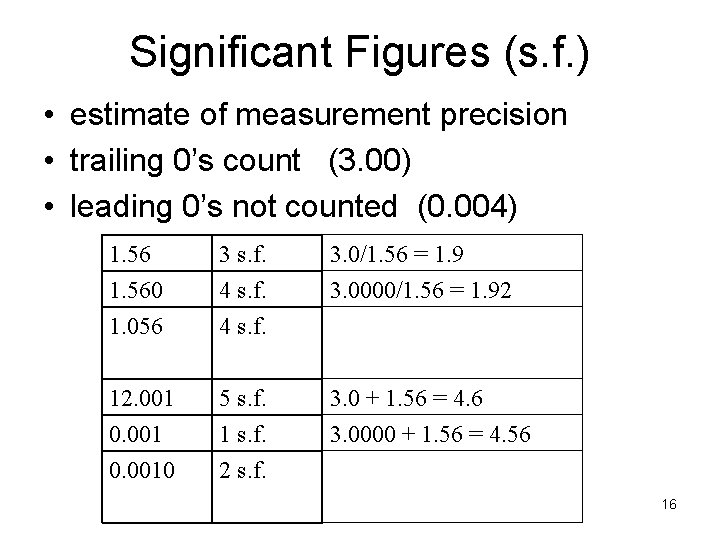
Significant Figures (s. f. ) • trailing 0’s count, e. g. 1. 560 • leading 0’s not counted, e. g. 0. 01 • more s. f. implies more precision 1. 560 1. 056 3 s. f. 4 s. f. 12. 001 0. 0010 5 s. f. 1 s. f. 2 s. f. 7

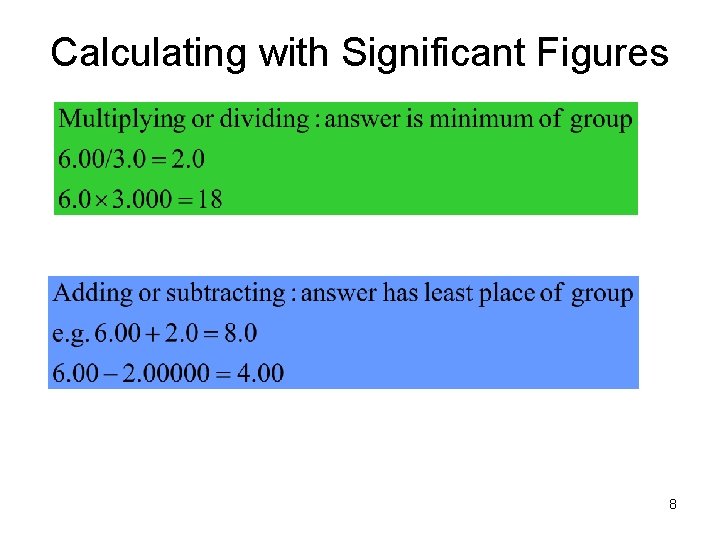
Calculating with Significant Figures 8

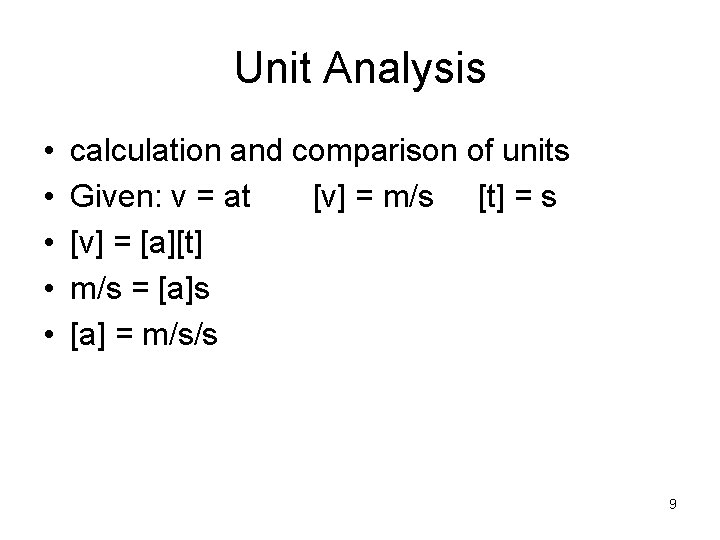
Unit Analysis • • • calculation and comparison of units Given: v = at [v] = m/s [t] = s [v] = [a][t] m/s = [a]s [a] = m/s/s 9

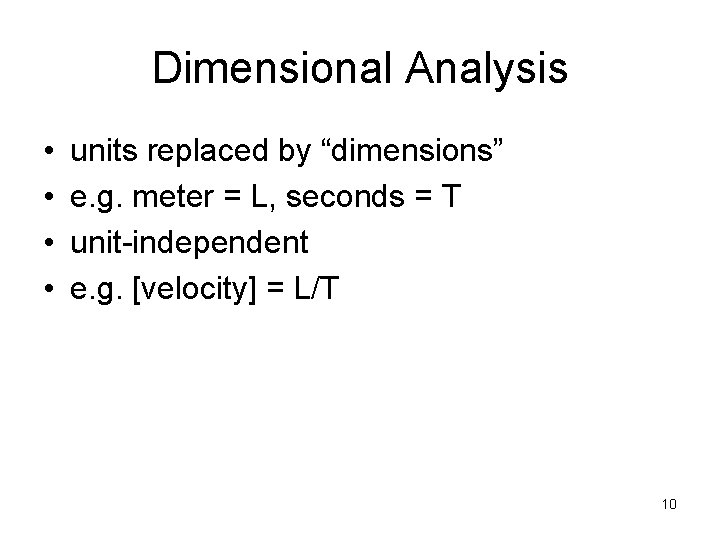
Dimensional Analysis • • units replaced by “dimensions” e. g. meter = L, seconds = T unit-independent e. g. [velocity] = L/T 10

Converting Units 11

Problem Solving • • write key data as you read mark unknowns with “? ” double check each value use SI values, e. g. 5 cm 0. 05 m sketch physical situation write equations solve, consider, read question again 12

Summary • • Unit and Dimensional Analysis Metric Prefixes -9 to +9 Unit Conversions Problem Solving 13

14

15

Significant Figures (s. f. ) • estimate of measurement precision • trailing 0’s count (3. 00) • leading 0’s not counted (0. 004) 1. 560 1. 056 3 s. f. 4 s. f. 3. 0/1. 56 = 1. 9 3. 0000/1. 56 = 1. 92 12. 001 0. 0010 5 s. f. 1 s. f. 2 s. f. 3. 0 + 1. 56 = 4. 6 3. 0000 + 1. 56 = 4. 56 16

Common Prefixes Prefix giga mega kilo centi milli micro nano Symbol G M k c m μ n Power of 10 109 106 103 10 -2 10 -3 10 -6 10 -9 17