Are topical NSAIDs a safe and effective treatment

















- Slides: 20

Are topical NSAIDs a safe and effective treatment for Corneal Abrasions? Department of Emergency Medicine University of Pennsylvania Health System Andrew Sheep, MD PGY-3 January 15 th, 2014

References • Congdon NG et al. Corneal complications associated with topical ophthalmic use of nonsteroidal anti-inflammatory drugs. J Cataract Refract Surg 2001; 27(4): 622– 631. • Szucs PA et al. Safety and efficacy of diclofenac ophthalmic solution in the treatment of corneal abrasions. Ann Emerg Med. 2000 Feb; 35(2): 131 -7. • Weaver CS, Terrell KM. Evidence-based emergency medicine. Update: do ophthalmic nonsteroidal anti-inflammatory drugs reduce the pain associated with simple corneal abrasion without delaying healing? Ann Emerg Med. 2003; 41: 134 -40 • Calder LA et al. Topical nonsteroidal anti-inflammatory drugs for corneal abrasions: meta-analysis of randomized trials. Acad Emerg Med. 2005 May; 12(5): 467 -73.

Congdon NG 2001 • Case reports from 1994 -2000 of complications of topical NSAIDs in corneal abrasion reported to the American Society of Cataract and Refractive Surgery • Cases were classified as "mild, " "moderate, " or "severe" according to predetermined clinical criteria. • 51 cases (36. 4%) were mild, 55 (39. 3%) moderate, and 34 (24. 3%) severe

Congdon NG 2001 • A mild case was defined as a condition limited to the corneal epithelium (persistent epithelial irritation) • A moderate case was characterized by loss or inflammation of corneal stromal substance (eg, stromal infiltration, ulceration, suppuration, or thinning) • A severe case had at least 1 of the following—wound leak, corneal perforation, use of tissue adhesive, or enucleation.

Congdon NG 2001 • Conclusion – Almost all cases of moderate and all cases of severe complications were in patients with comorbid DM, preexisting ocular pathology, or prolonged (>48 hours) use. • Limitations: – This is a series of case reports – Many of these patients had ocular surgery – Many patients used it >48 hours

Szucs PA 2000 • Szucs PA et al. Safety and efficacy of diclofenac ophthalmic solution in the treatment of corneal abrasions. Ann Emerg Med. 2000 Feb; 35(2): 131 -7.

Szucs PA 2000 • Prospective, randomized, double-blinded, placebocontrolled clinical trial of consecutive patients to a community ED, receiving either topical NSAID or placebo for corneal abrasion • 25 received diclofenac and 24 received placebo drops. Both groups were similar in gender, age, pretreatment pain duration, Numeric Pain Intensity Scale (NPIS) , and analgesic use.

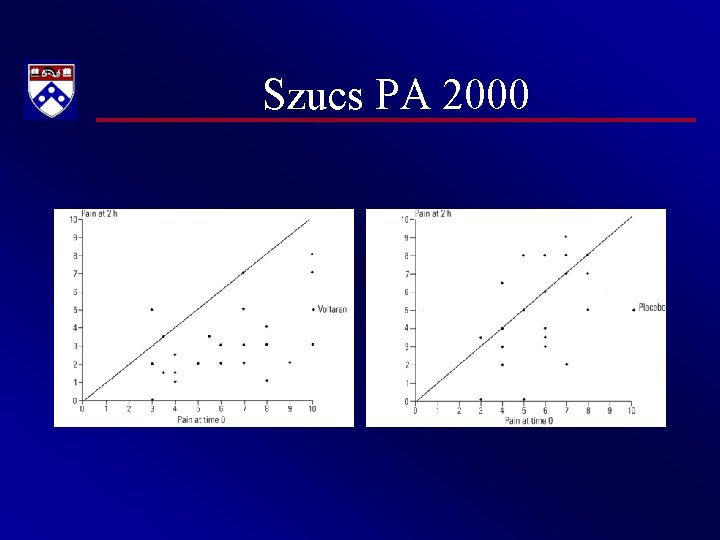
Szucs PA 2000 • There was significantly greater improvement in the 2 -hour mean NPIS score in the diclofenac group 3. 1 (95% CI 2. 3 to 4. 0; Figure 1) compared with the control group 1. 0 (95% CI 0. 1 to 2. 0; P=. 002; Figure 2).

Szucs PA 2000

Szucs PA 2000 • Patients in the diclofenac group were half as likely to take oxycodone-acetaminophen rescue medication (20% of diclofenac patients [95% CI 4% to 36%] versus 42% of control vehicle patients [95% CI 22% to 62%]), although this was not statistically significant.

Szucs PA 2000 • No reported complications associated with shortterm diclofenac or placebo use. • No patients (0/49) followed for up 10 days developed a corneal infection during the study, and all reported complete resolution of pain and return to baseline visual acuity within 1 week of treatment.

Weaver CS 2003 • Weaver CS, Terrell KM. Evidence-based emergency medicine. Update: do ophthalmic nonsteroidal anti-inflammatory drugs reduce the pain associated with simple corneal abrasion without delaying healing? Ann Emerg Med. 2003; 41: 134 -40

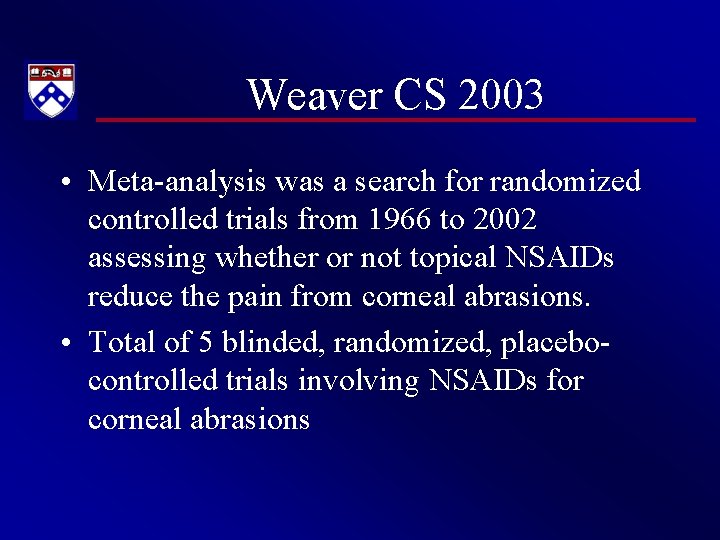
Weaver CS 2003 • Meta-analysis was a search for randomized controlled trials from 1966 to 2002 assessing whether or not topical NSAIDs reduce the pain from corneal abrasions. • Total of 5 blinded, randomized, placebocontrolled trials involving NSAIDs for corneal abrasions

Weaver CS 2003 • The qualitative summary indicates that NSAIDs provide greater pain relief and improvement of other subjective symptoms when compared with placebo • The use of ophthalmic NSAIDs may decrease the need for sedating analgesics. • However, they’re more expensive than orals

Weaver CS 2003 • In clinical practice, the evidence gained from clinical trials must be placed in the context of clinician experience. • Patients who can afford the medication, must return to work immediately, and for whom potential opioid-induced sedation is intolerable are the most likely candidates for the administration of ophthalmic NSAIDs during the first 24 hours after a traumatic corneal abrasion.

Calder LA 2005 • Calder LA et al. Topical nonsteroidal antiinflammatory drugs for corneal abrasions: meta-analysis of randomized trials. Acad Emerg Med. 2005 May; 12(5): 467 -73.

Calder LA 2005 • This was a systematic literature review and metaanalysis of randomized clinical trials. • Outcomes were pain scale scores at 24 hours and adverse effects. • 11 RCTs met inclusion criteria. • Seven trials enrolled fewer than 100 patients, and more than half of the studies were conducted in Europe. Five trials reported suitable data for analysis.

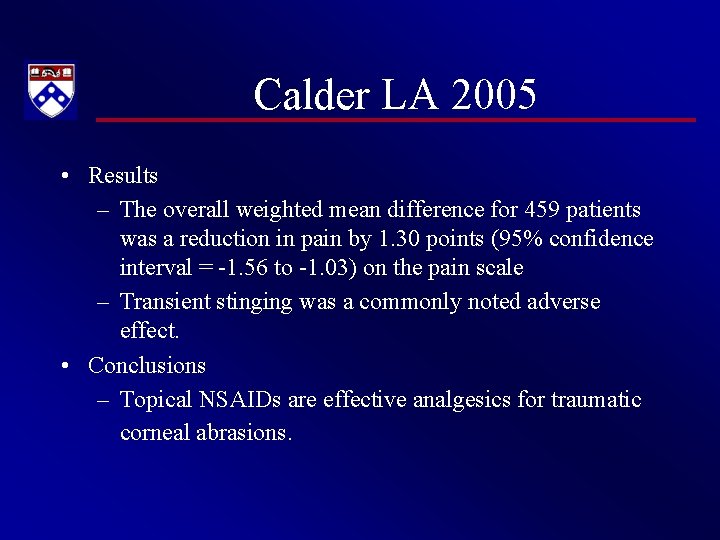
Calder LA 2005 • Results – The overall weighted mean difference for 459 patients was a reduction in pain by 1. 30 points (95% confidence interval = -1. 56 to -1. 03) on the pain scale – Transient stinging was a commonly noted adverse effect. • Conclusions – Topical NSAIDs are effective analgesics for traumatic corneal abrasions.

HUPism • Topical NSAIDs such as diclofenac and ketorolac are safe and effective in uncomplicated corneal abrasion for acute and short-term pain relief, and can be considered as sole analgesic. • They should not be used in patients with preexisting ocular pathology. • They should not be used greater than 24 -48 hours.

Thanks!
 Insidan region jh
Insidan region jh Dehydration gastritis
Dehydration gastritis Miosis
Miosis Nsaid classification
Nsaid classification Safe feed safe food
Safe feed safe food Safe people safe places
Safe people safe places Principles effective addiction treatment
Principles effective addiction treatment Cold cream definition in pharmacy
Cold cream definition in pharmacy Medication administration 1 pretest
Medication administration 1 pretest Six rights of medication administration
Six rights of medication administration Order literature review
Order literature review Collodion dosage form
Collodion dosage form Two skin protective agents
Two skin protective agents Syllabus types
Syllabus types Topical scope
Topical scope Topical dosage forms
Topical dosage forms Situational syllabus example
Situational syllabus example Topical vocabulary
Topical vocabulary Topical approach meaning
Topical approach meaning Pemberian obat secara topikal
Pemberian obat secara topikal Be prepared sermon
Be prepared sermon