Answers from Whats Hot 1 U is not









- Slides: 25

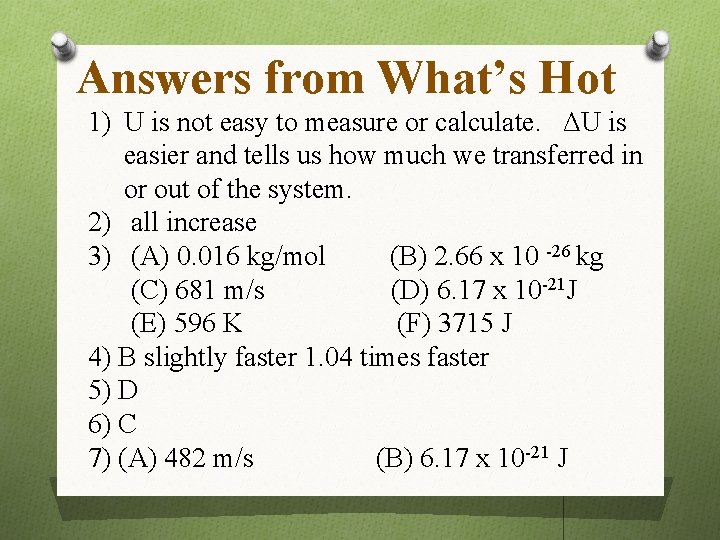
Answers from What’s Hot 1) U is not easy to measure or calculate. ΔU is easier and tells us how much we transferred in or out of the system. 2) all increase 3) (A) 0. 016 kg/mol (B) 2. 66 x 10 -26 kg (C) 681 m/s (D) 6. 17 x 10 -21 J (E) 596 K (F) 3715 J 4) B slightly faster 1. 04 times faster 5) D 6) C 7) (A) 482 m/s (B) 6. 17 x 10 -21 J

Temperature and Heat Linear Expansion and Heat Transfer

Today we will learn about… 2. Heat transfer and thermal expansion Students should understand heat transfer and thermal expansion, so they can: a) Calculate how the flow of heat through a slab of material is affected by changes in the thickness or area of the slab, or the temperature difference between the two faces of the slab. b) Analyze what happens to the size and shape of an object when it is heated. c) Analyze qualitatively the effects of conduction, radiation, and convection in thermal processes

Temperature and Expansion So why expansion and contraction? As a substance is heated the molecules move or vibrate faster. They hit each other harder causing the particles to spread farther apart. In gases it results in higher pressure and greater volume, which we will investigate in greater detail in the next presentation. Liquids and solids are held together by strong intermolecular forces, so there is a only a slight expansion. The expansion of liquids and solids is important since it can tear apart the things we build.

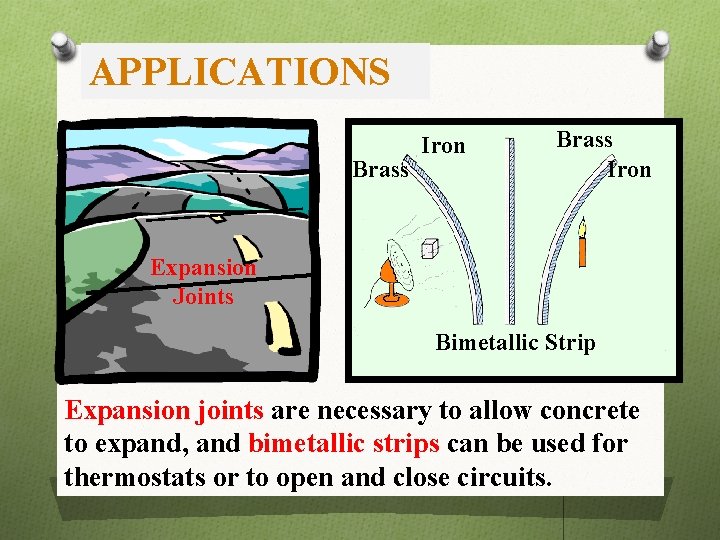
APPLICATIONS Brass Iron Expansion Joints Bimetallic Strip Expansion joints are necessary to allow concrete to expand, and bimetallic strips can be used for thermostats or to open and close circuits.

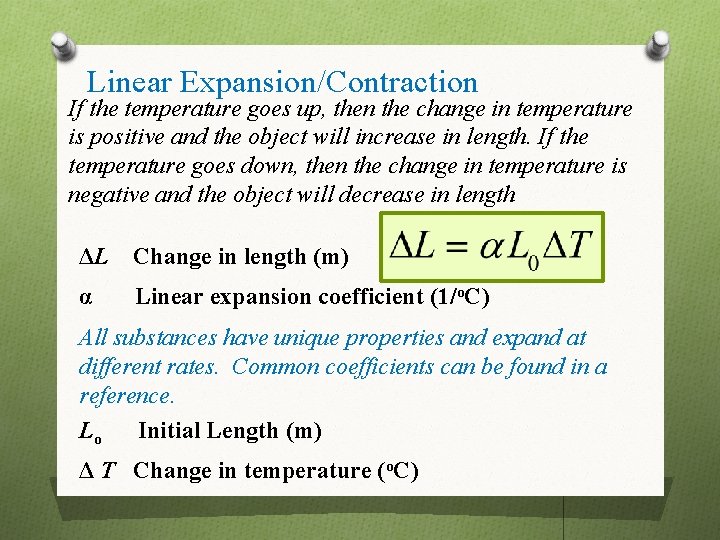
Linear Expansion/Contraction If the temperature goes up, then the change in temperature is positive and the object will increase in length. If the temperature goes down, then the change in temperature is negative and the object will decrease in length ΔL Change in length (m) α Linear expansion coefficient (1/o. C) All substances have unique properties and expand at different rates. Common coefficients can be found in a reference. Lo Initial Length (m) Δ T Change in temperature (o. C)

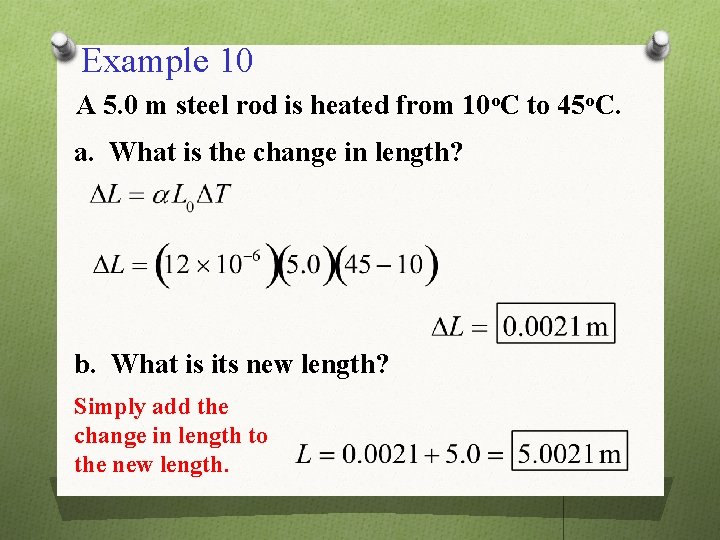
Example 10 A 5. 0 m steel rod is heated from 10 o. C to 45 o. C. a. What is the change in length? b. What is its new length? Simply add the change in length to the new length.

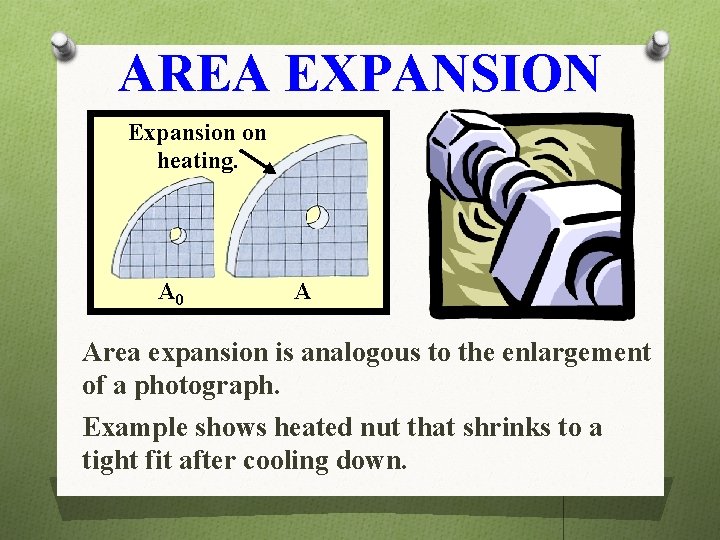
AREA EXPANSION Expansion on heating. A 0 A Area expansion is analogous to the enlargement of a photograph. Example shows heated nut that shrinks to a tight fit after cooling down.

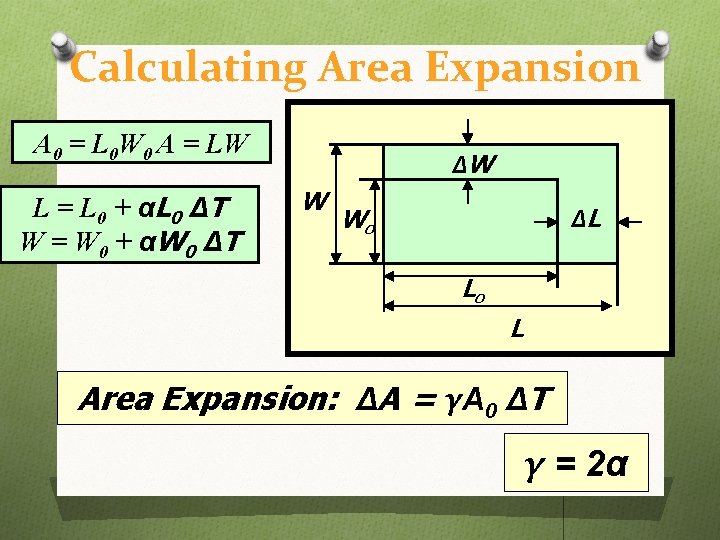
Calculating Area Expansion A 0 = L 0 W 0 A = LW L = L 0 + αL 0 Δ T W = W 0 + αW 0 Δ T ΔW W ΔL Wo Lo L Area Expansion: ΔA = γΑ 0 ΔT γ = 2α

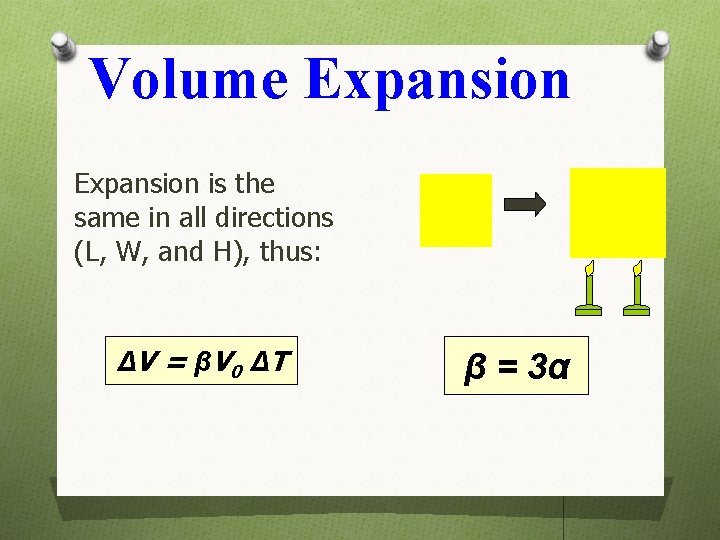
Volume Expansion is the same in all directions (L, W, and H), thus: ΔV = β V 0 ΔT β = 3α


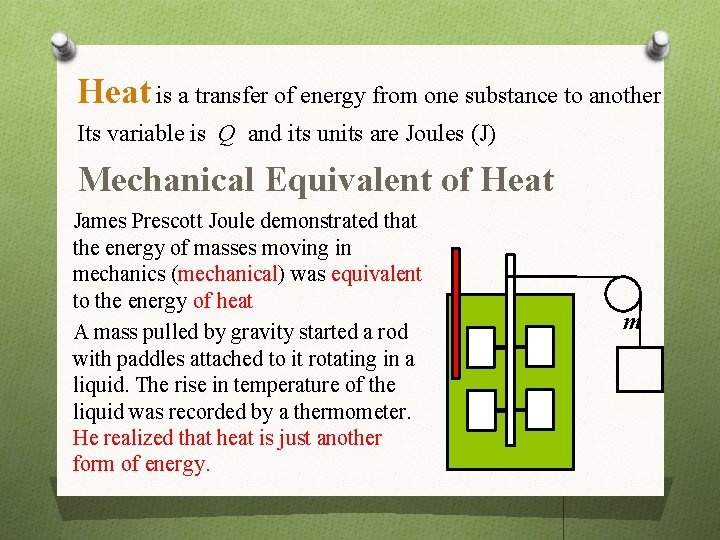
Heat is a transfer of energy from one substance to another Its variable is Q and its units are Joules (J) Mechanical Equivalent of Heat James Prescott Joule demonstrated that the energy of masses moving in mechanics (mechanical) was equivalent to the energy of heat A mass pulled by gravity started a rod with paddles attached to it rotating in a liquid. The rise in temperature of the liquid was recorded by a thermometer. He realized that heat is just another form of energy. m

JAMES PRESCOTT j. OULE https: //www. youtube. com/watch? v=f 9 e. Vag. CUPAE

Zeroth Law of Thermodynamics O Two objects in thermal equilibrium with each other are at the same temperature O If objects A and B are separately in thermal equilibrium with a third object, C, then A and B are in thermal equilibrium with each other.

Implications of the “ 0” Law of Thermo • When hot touches cold, then the hot particles collide with the cold and transfer some of their energy. The hot object losses energy to the cold object and it slows and cools. • When they reach thermal equilibrium (equal temperature) the process of heat transfer stops. • The natural direction of heat transfer has to be from hot to cold.

Methods of Heat Transfer O Need to know the rate at which energy is transferred O Need to know the mechanisms responsible for the transfer O Methods include O Conduction O Convection O Radiation

Kinds of Heat Transfer Consider the operation of a typical coffee maker: Think about how heat is transferred by: Conduction? Convection? Radiation?

Conduction O The transfer can be viewed on an atomic scale O Less energetic particles gain energy during collisions with more energetic particles O Rate of conduction depends upon the characteristics of the substance. O In general, metals are good conductors O They contain large numbers of electrons that are relatively free to move through the metal O Conduction can occur only if there is a difference in temperature

Convection O Energy transferred by the movement of a fluid O Gasses and liquids O When the movement results from differences in density, it is called natural convection O When the movement is forced by a fan or a pump, it is called forced convection

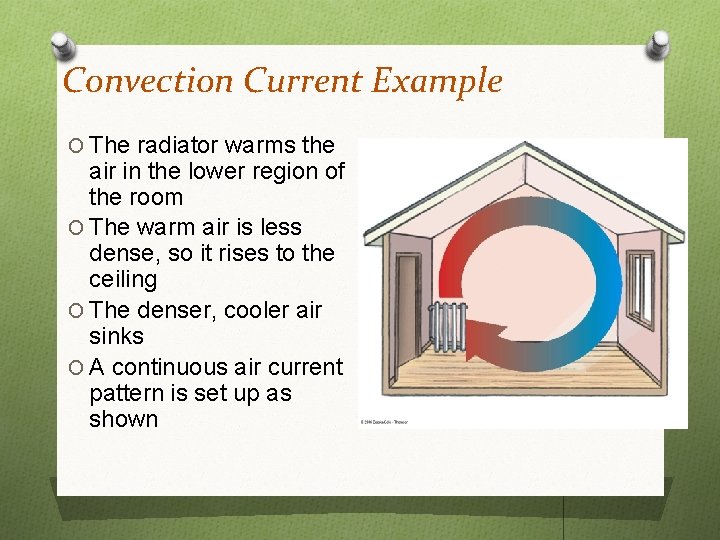
Convection Current Example O The radiator warms the air in the lower region of the room O The warm air is less dense, so it rises to the ceiling O The denser, cooler air sinks O A continuous air current pattern is set up as shown

Radiation O Radiation does not require physical contact O All objects radiate energy continuously in the form of electromagnetic waves due to thermal vibrations of the molecules O Rate of radiation is given by Stefan’s Law

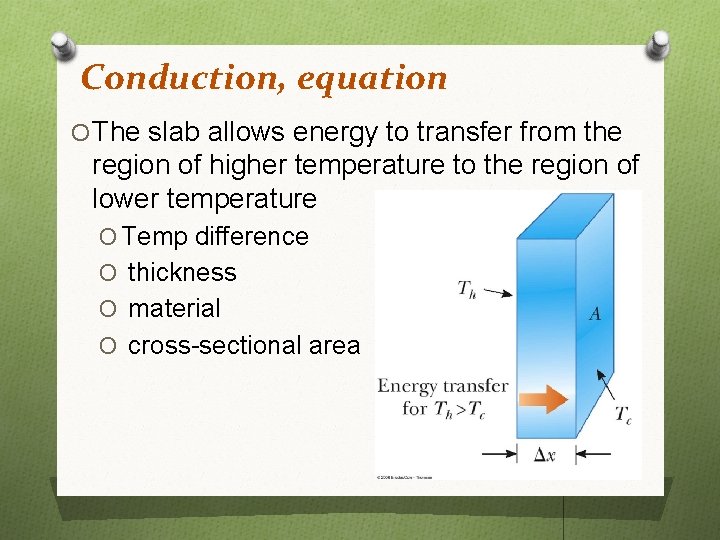
Conduction, equation O The slab allows energy to transfer from the region of higher temperature to the region of lower temperature O Temp difference O thickness O material O cross-sectional area

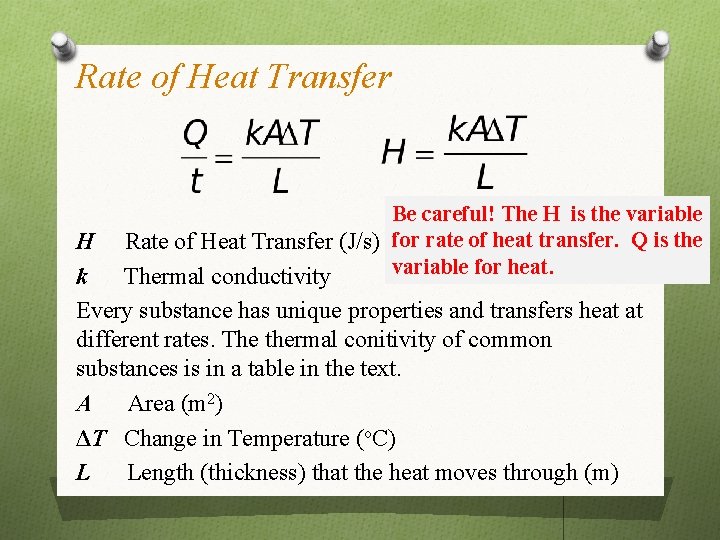
Rate of Heat Transfer Be careful! The H is the variable Rate of Heat Transfer (J/s) for rate of heat transfer. Q is the variable for heat. Thermal conductivity H k Every substance has unique properties and transfers heat at different rates. The thermal conitivity of common substances is in a table in the text. A Area (m 2) ΔT Change in Temperature (o. C) L Length (thickness) that the heat moves through (m)


Example 14 A frying pan made of Aluminum sits on top of a burner on the stove. Assume heat is delivered evenly over the bottom surface of the pan. The pan has a radius of 10 cm and is 0. 3 cm thick. When the stove is turned on the top of the pan is at 25 o. C and the bottom of the pan is at 200 o. C. How much heat transfers to the food every 10 seconds. Q = 4. 40 x 106 J
 Whats hot whats not
Whats hot whats not Not genuine, not true, not valid
Not genuine, not true, not valid White hot vs red hot temperature
White hot vs red hot temperature Hot working diagram
Hot working diagram Perbedaan hot lava dan hot lava volcano
Perbedaan hot lava dan hot lava volcano Be either hot or cold
Be either hot or cold Hot or not composite images
Hot or not composite images Do not make friends with a hot tempered man
Do not make friends with a hot tempered man Energy bar chart example
Energy bar chart example Khan academy least common denominator
Khan academy least common denominator Whats not included in gdp
Whats not included in gdp If you are not confused you're not paying attention
If you are not confused you're not paying attention Informal-casual
Informal-casual Attention is not explanation
Attention is not explanation Being too broad
Being too broad If p, then q
If p, then q Just about right scale
Just about right scale Love is not all: it is not meat nor drink
Love is not all: it is not meat nor drink Ears that hear and eyes that see
Ears that hear and eyes that see Pp sit
Pp sit You can't improve what you don't measure quote
You can't improve what you don't measure quote We will not be moved when everything around is shaking
We will not be moved when everything around is shaking Not a rustling leaf, not a bird in flight
Not a rustling leaf, not a bird in flight You can not not communicate
You can not not communicate Task #2 equation problems answers
Task #2 equation problems answers Combining half equations chemsheets
Combining half equations chemsheets