Anglo Research A Division of Anglo Operations Limited







- Slides: 25

Anglo Research A Division of Anglo Operations Limited Thermodynamics & Speciation H 2 SO 4 -Me(II)SO 4 -H 2 O, T 200 C Johann Steyl SAIMM Hydrometallurgy Conference 26 February 2009

Agenda • Introduction • Model Development - First principles perspective (infinite dilution) - Phenomenological description (real solutions) • Application 2

Introduction Why solution chemistry model? • Kinetic processes influenced by solution species (entropy driven) • Thermodynamic properties indirectly influence kinetics, for example, − Solvent activity • Anticipate continuous circuit response to step change (incorporate into steady-state model of circuit) 3

Introduction Modelling Methodology • Interaction versus Speciation • Combine interaction/speciation (modern approach) − Must be guided by minimum parameter space − Chemical kinetics (explicitly recognise only contact pairs, e. g. HSO 4−) − Pitzer interaction-type model provides strong enough framework − Surrogate salt approach (Mg) • Predict speciation at high temperatures − Regress model to speciation data at room temperature − Regress model to thermodynamic data over full T & [Me] range − Take care in selecting K , ΔH , ΔCp at the reference state 4

Agenda • Introduction • Model Development - First principles perspective (infinite dilution) 5

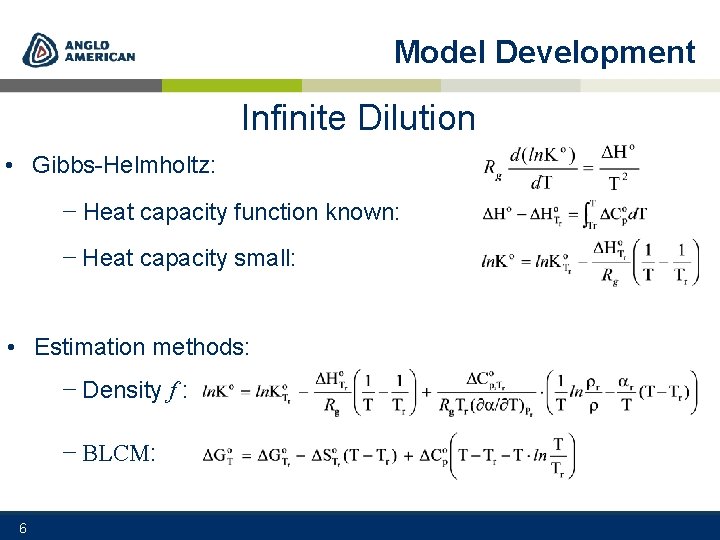
Model Development Infinite Dilution • Gibbs-Helmholtz: − Heat capacity function known: − Heat capacity small: • Estimation methods: − Density f : − BLCM: 6

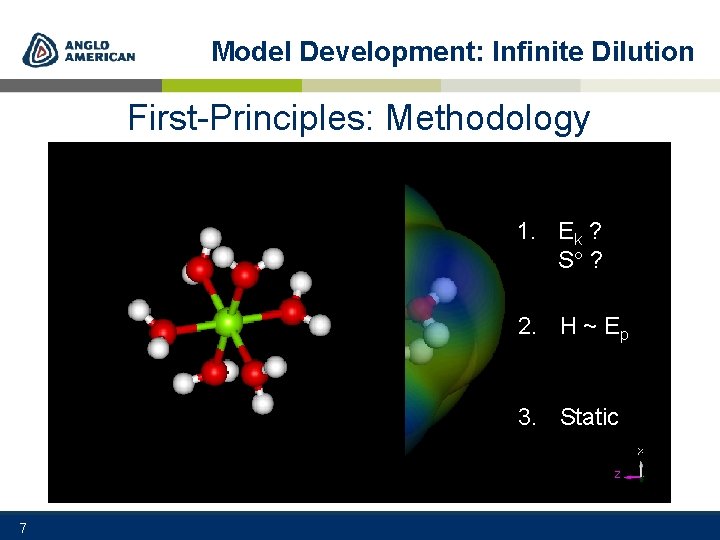
Model Development: Infinite Dilution First-Principles: Methodology DMol 3/COSMO 1. Ek ? S ? 2. H ~ Ep 3. Static 7

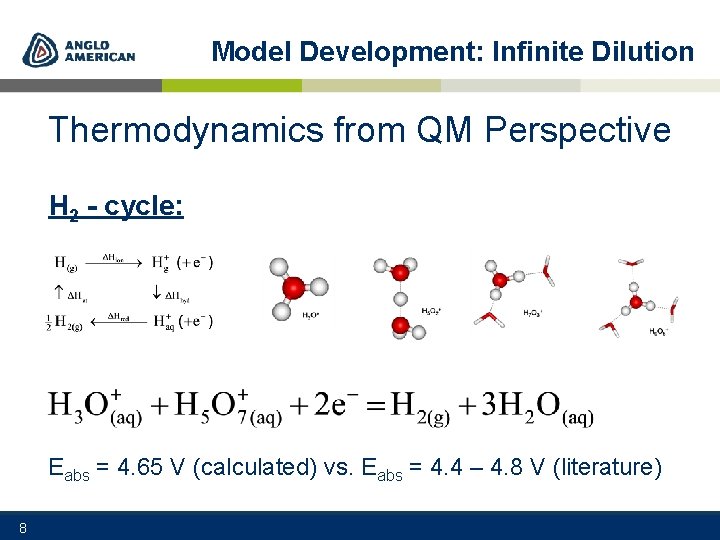
Model Development: Infinite Dilution Thermodynamics from QM Perspective H 2 - cycle: Eabs = 4. 65 V (calculated) vs. Eabs = 4. 4 – 4. 8 V (literature) 8

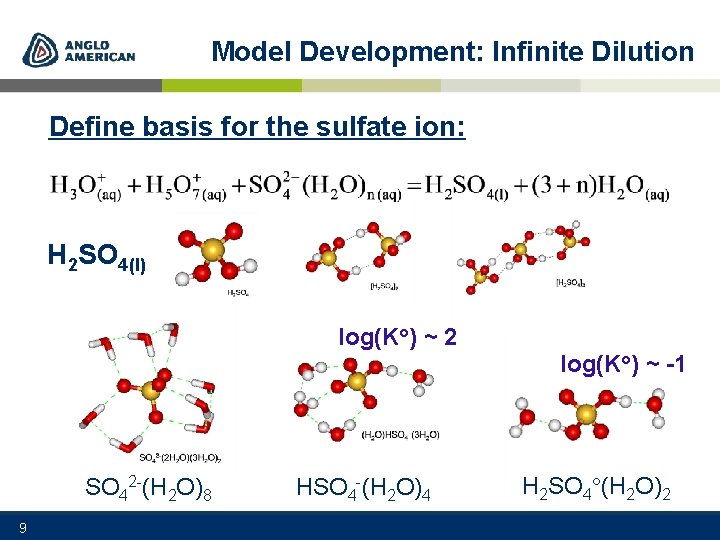
Model Development: Infinite Dilution Define basis for the sulfate ion: H 2 SO 4(l) log(K ) ~ 2 log(K ) ~ -1 SO 42 -(H 2 O)8 9 HSO 4 -(H 2 O)4 H 2 SO 4 (H 2 O)2

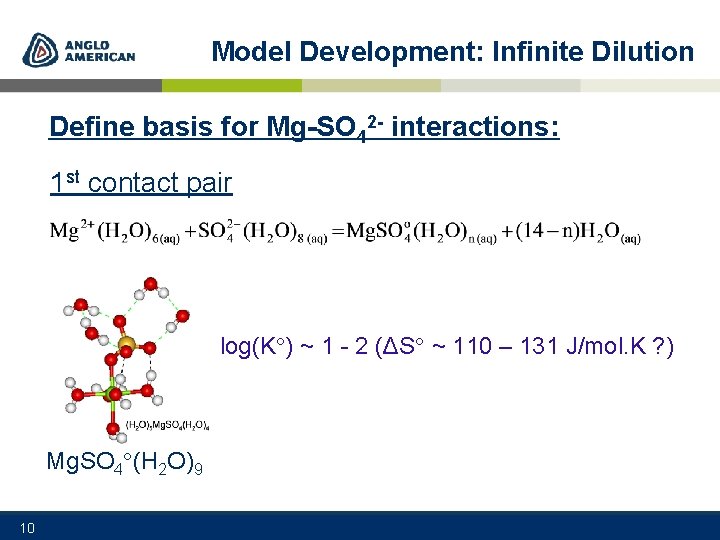
Model Development: Infinite Dilution Define basis for Mg-SO 42 - interactions: 1 st contact pair log(K ) ~ 1 - 2 (ΔS ~ 110 – 131 J/mol. K ? ) Mg. SO 4 (H 2 O)9 10

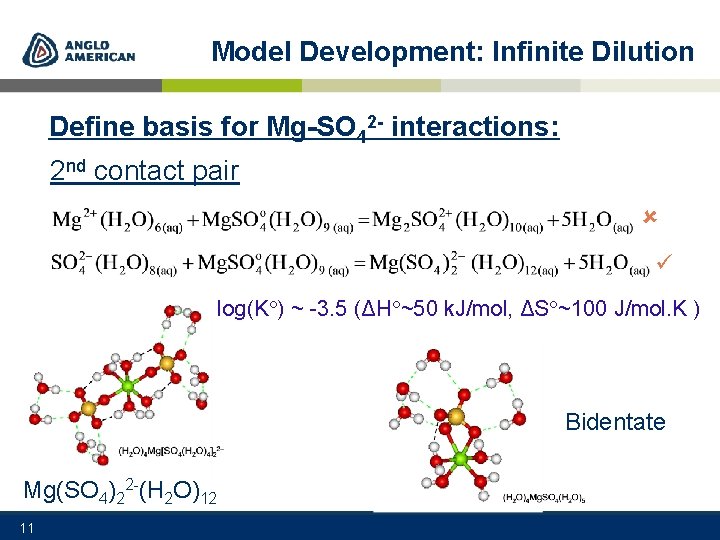
Model Development: Infinite Dilution Define basis for Mg-SO 42 - interactions: 2 nd contact pair log(K ) ~ -3. 5 (ΔH ~50 k. J/mol, ΔS ~100 J/mol. K ) Bidentate Mg(SO 4)22 -(H 2 O)12 11

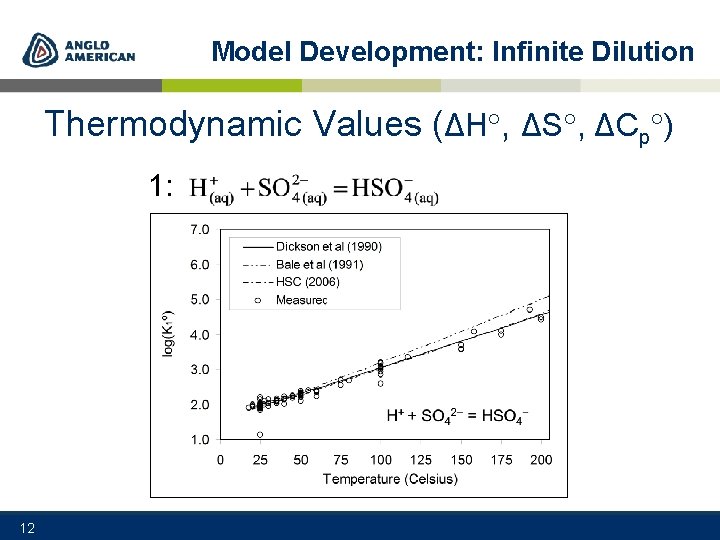
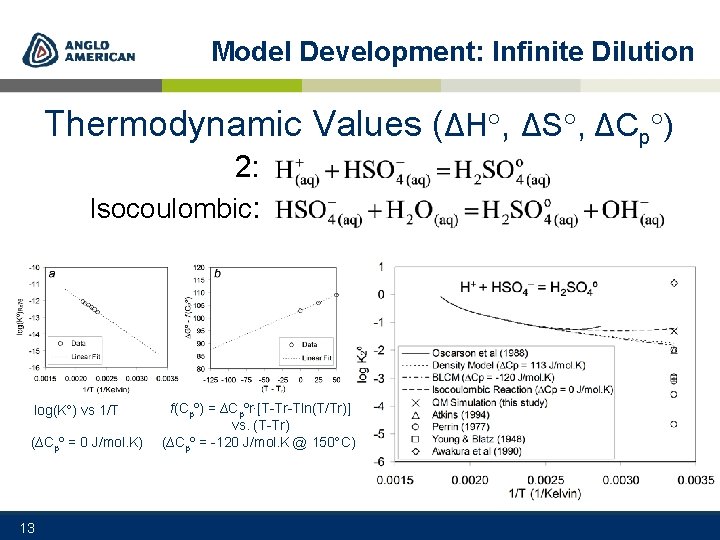
Model Development: Infinite Dilution Thermodynamic Values (ΔH , ΔS , ΔCp ) 1: 12

Model Development: Infinite Dilution Thermodynamic Values (ΔH , ΔS , ΔCp ) 2: Isocoulombic: log(K ) vs 1/T (∆Cpº = 0 J/mol. K) 13 f(Cpº) = ∆Cpºr∙[T-Tr-Tln(T/Tr)] vs. (T-Tr) (∆Cpº = -120 J/mol. K @ 150 C)

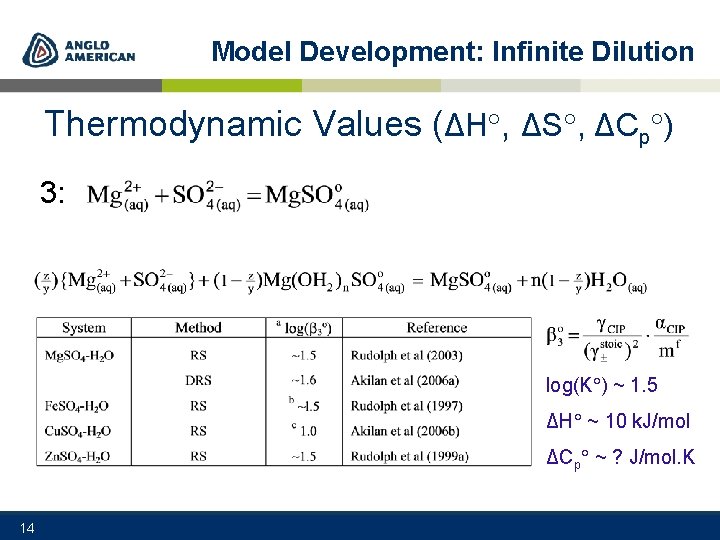
Model Development: Infinite Dilution Thermodynamic Values (ΔH , ΔS , ΔCp ) 3: log(K ) ~ 1. 5 ΔH ~ 10 k. J/mol ΔCp ~ ? J/mol. K 14

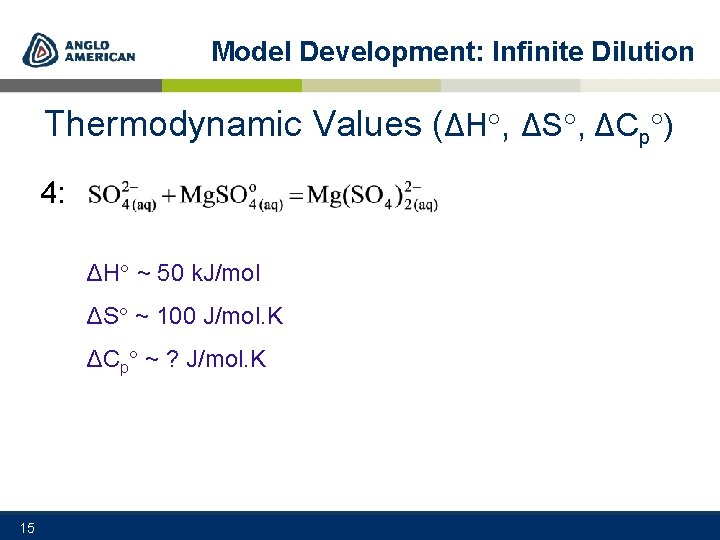
Model Development: Infinite Dilution Thermodynamic Values (ΔH , ΔS , ΔCp ) 4: ΔH ~ 50 k. J/mol ΔS ~ 100 J/mol. K ΔCp ~ ? J/mol. K 15

Agenda • Introduction • Model Development - First principles perspective (infinite dilution) - Phenomenological description (real solutions) 16

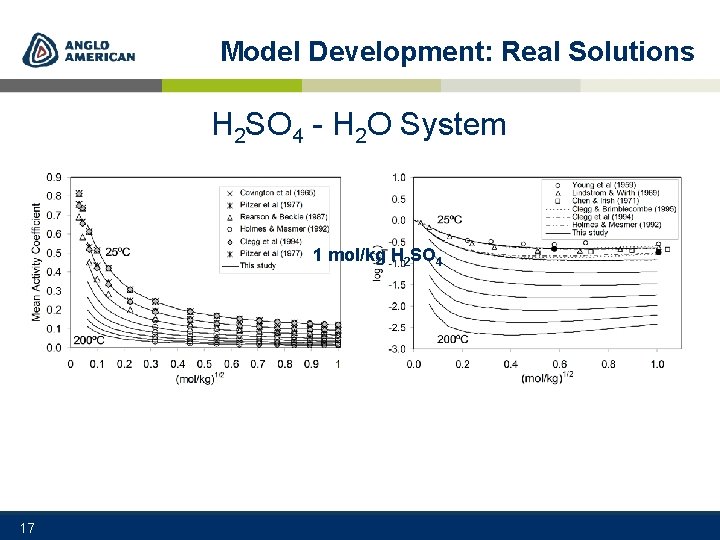
Model Development: Real Solutions H 2 SO 4 - H 2 O System 1 mol/kg H 2 SO 4 17

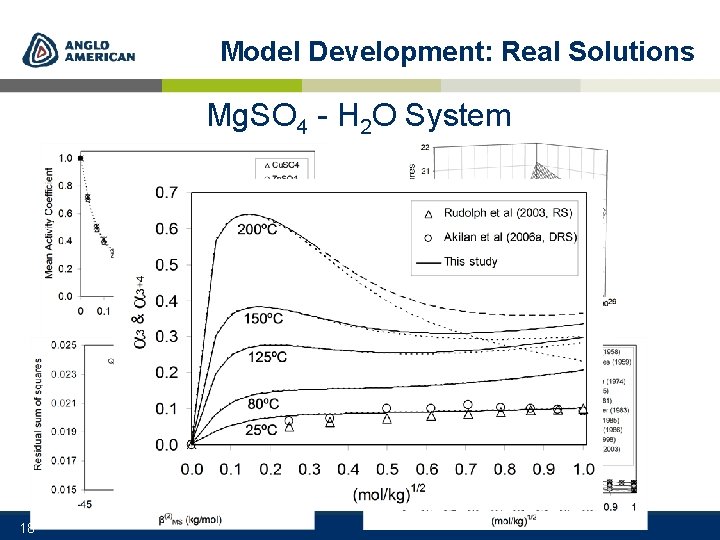
Model Development: Real Solutions Mg. SO 4 - H 2 O System era Lit e tur 18

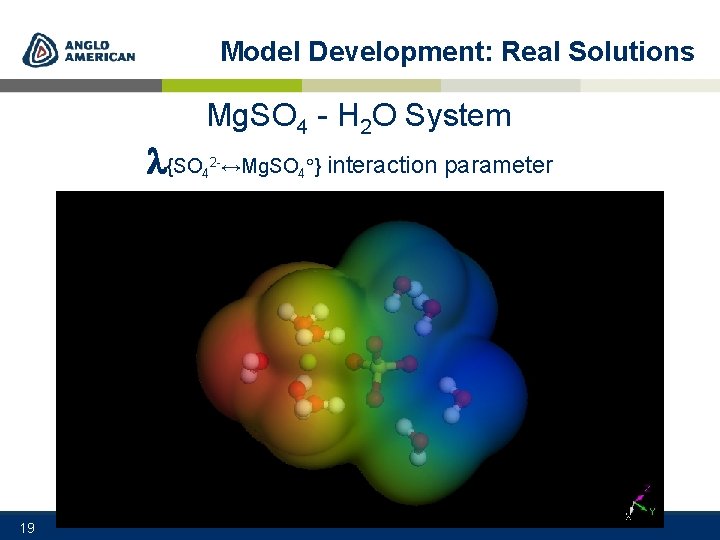
Model Development: Real Solutions {SO 19 Mg. SO 4 - H 2 O System 4 2 -↔Mg. SO 4 } interaction parameter

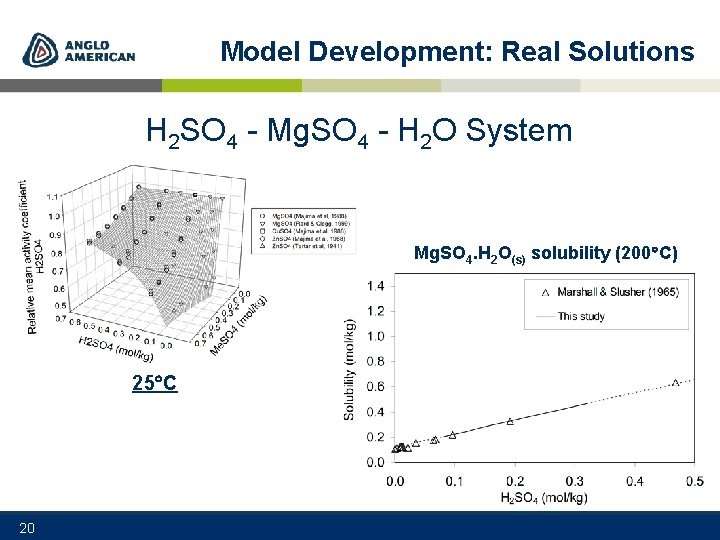
Model Development: Real Solutions H 2 SO 4 - Mg. SO 4 - H 2 O System Mg. SO 4. H 2 O(s) solubility (200 C) 25 C 20

Agenda • Introduction • Model Development - First principles perspective (infinite dilution) - Phenomenological description (real solutions) • Application 21

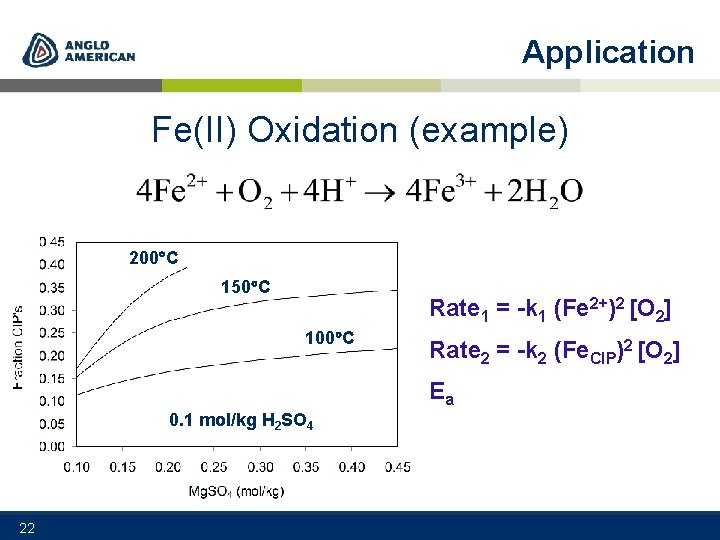
Application Fe(II) Oxidation (example) 200 C 150 C Rate 1 = -k 1 (Fe 2+)2 [O 2] 100 C Rate 2 = -k 2 (Fe. CIP)2 [O 2] Ea 0. 1 mol/kg H 2 SO 4 22

Acknowledgements Anglo American plc Paul Dempsey Anglo Research Dr. Maggie Burger 23

? 24

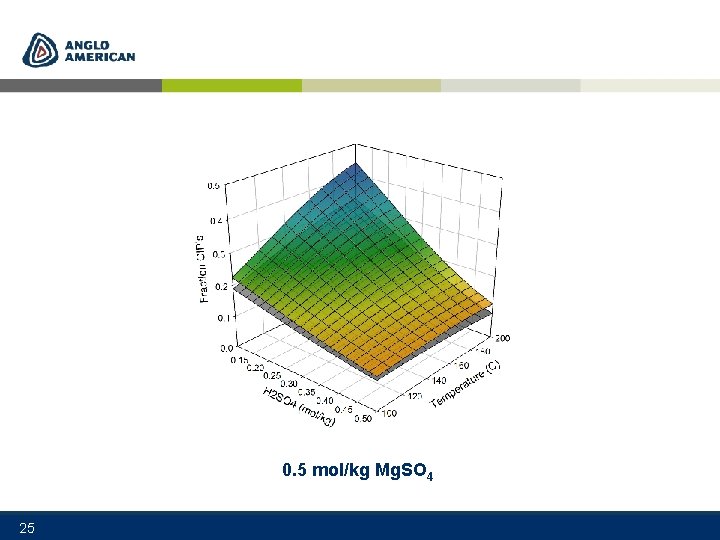
0. 5 mol/kg Mg. SO 4 25
 Ficks law
Ficks law Ecc division of larsen & toubro
Ecc division of larsen & toubro 369 times 2
369 times 2 Synthetic division pattern
Synthetic division pattern H.c.f
H.c.f Synthetic divison
Synthetic divison Genome research limited
Genome research limited Genome research limited
Genome research limited Scope of operations research
Scope of operations research Scope of operation research
Scope of operation research Deterministic operations research
Deterministic operations research Markov chain operations research
Markov chain operations research Scope of operation research
Scope of operation research Operations research
Operations research Taha operations research
Taha operations research Industrielogistik leoben
Industrielogistik leoben Operations research
Operations research Georgia tech operations research
Georgia tech operations research Rumus kriteria laplace
Rumus kriteria laplace Significance of operations research
Significance of operations research Linear programming in operation research
Linear programming in operation research Ibm research division
Ibm research division Anglo-conformity
Anglo-conformity Anglo sakson hukuk sistemi
Anglo sakson hukuk sistemi Anglo saxons characteristics
Anglo saxons characteristics A metaphorical phrase used to replace a concrete noun
A metaphorical phrase used to replace a concrete noun