AN INTRODUCTION TO THE CHEMISTRY OF ALKANES KNOCKHARDY










- Slides: 28

AN INTRODUCTION TO THE CHEMISTRY OF ALKANES KNOCKHARDY PUBLISHING

KNOCKHARDY PUBLISHING THE CHEMISTRY OF ALKANES INTRODUCTION This Powerpoint show is one of several produced to help students understand selected topics at AS and A 2 level Chemistry. It is based on the requirements of the AQA and OCR specifications but is suitable for other examination boards. Individual students may use the material at home for revision purposes or it may be used for classroom teaching if an interactive white board is available. Accompanying notes on this, and the full range of AS and A 2 topics, are available from the KNOCKHARDY SCIENCE WEBSITE at. . . www. argonet. co. uk/users/hoptonj/sci. htm Navigation is achieved by. . . either clicking on the grey arrows at the foot of each page or using the left and right arrow keys on the keyboard

THE CHEMISTRY OF ALKANES CONTENTS • Structure of alkanes • Physical properties of alkanes • Chemical properties of alkanes • Breaking covalent bonds • Chlorination via free radical substitution • Cracking • Revision check list

THE CHEMISTRY OF ALKANES Before you start it would be helpful to… • Recall the definition of a covalent bond • Be able to balance simple equations • Be able to write out structures for hydrocarbons

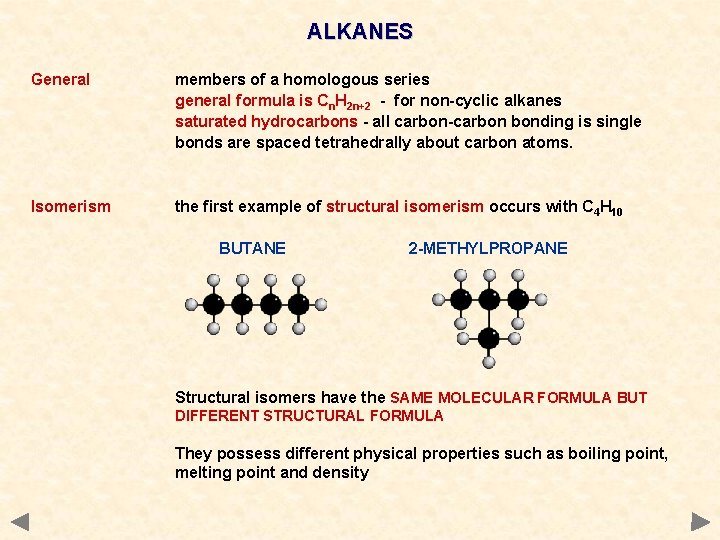
ALKANES General members of a homologous series general formula is Cn. H 2 n+2 - for non-cyclic alkanes saturated hydrocarbons - all carbon-carbon bonding is single bonds are spaced tetrahedrally about carbon atoms. Isomerism the first example of structural isomerism occurs with C 4 H 10 BUTANE 2 -METHYLPROPANE Structural isomers have the SAME MOLECULAR FORMULA BUT DIFFERENT STRUCTURAL FORMULA They possess different physical properties such as boiling point, melting point and density

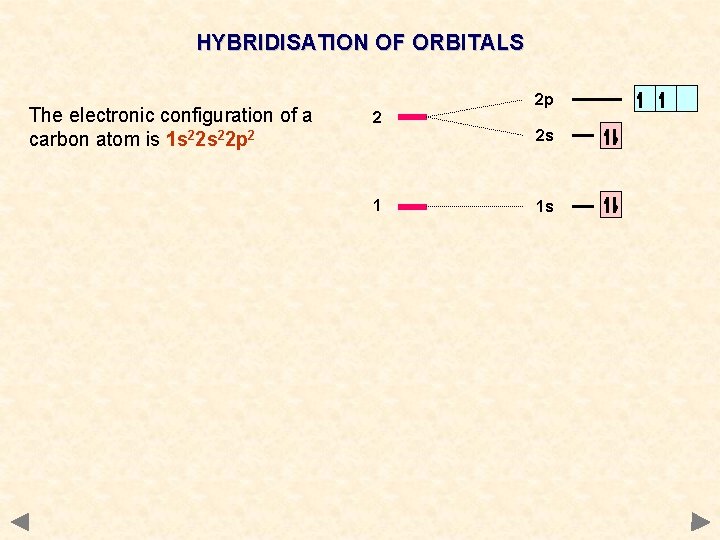
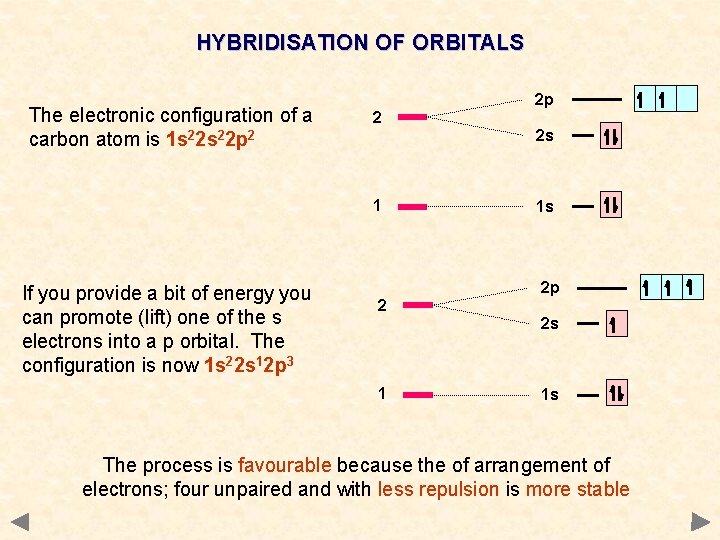
HYBRIDISATION OF ORBITALS The electronic configuration of a carbon atom is 1 s 22 p 2 2 2 p 2 s 1 1 s

HYBRIDISATION OF ORBITALS The electronic configuration of a carbon atom is 1 s 22 p 2 2 2 s 1 If you provide a bit of energy you can promote (lift) one of the s electrons into a p orbital. The configuration is now 1 s 22 s 12 p 3 2 p 2 1 s 2 p 2 s 1 1 s The process is favourable because the of arrangement of electrons; four unpaired and with less repulsion is more stable

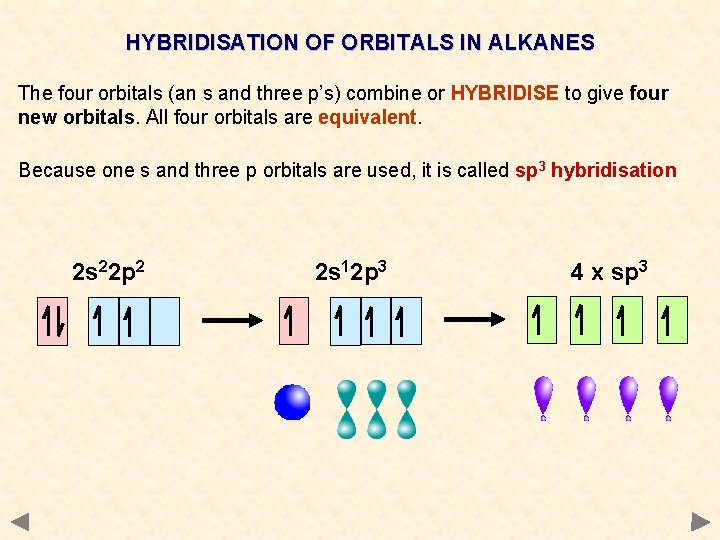
HYBRIDISATION OF ORBITALS IN ALKANES The four orbitals (an s and three p’s) combine or HYBRIDISE to give four new orbitals. All four orbitals are equivalent. Because one s and three p orbitals are used, it is called sp 3 hybridisation 2 s 22 p 2 2 s 12 p 3 4 x sp 3

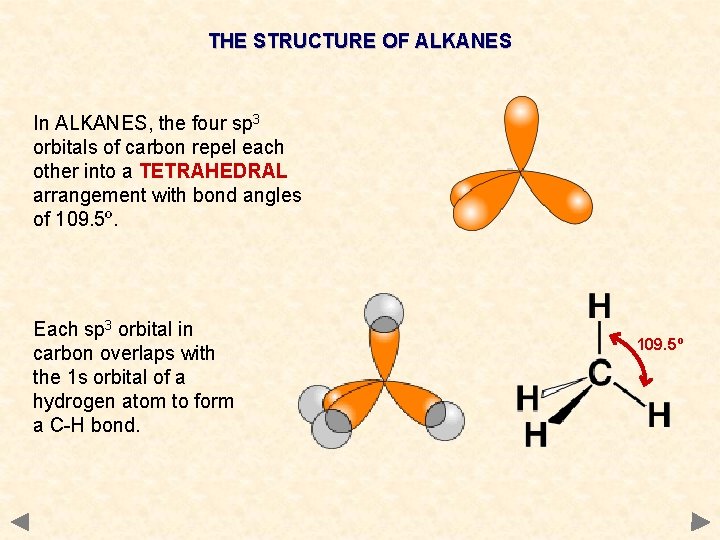
THE STRUCTURE OF ALKANES In ALKANES, the four sp 3 orbitals of carbon repel each other into a TETRAHEDRAL arrangement with bond angles of 109. 5º. Each sp 3 orbital in carbon overlaps with the 1 s orbital of a hydrogen atom to form a C-H bond. 109. 5º

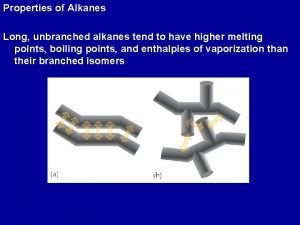
PHYSICAL PROPERTIES OF ALKANES Boiling point increases as they get more carbon atoms in their formula more atoms = greater intermolecular Van der Waals’ forces greater intermolecular force = more energy to separate the molecules greater energy required = higher boiling point CH 4 (-161°C) C 2 H 6 (-88°C) C 3 H 8 (-42°C) C 4 H 10 (-0. 5°C) difference gets less - mass increases by a smaller percentage straight chains molecules have greater interaction than branched “The greater the branching, the lower the boiling point” Melting point general increase with molecular mass the trend is not as regular as that for boiling point. Solubilityalkanes are non-polar so are immiscible with water they are soluble in most organic solvents.

CHEMICAL PROPERTIES OF ALKANES Introduction - fairly unreactive; (old family name, paraffin, meant little reactivity) - have relatively strong, almost NON-POLAR, SINGLE covalent bonds - they have no real sites that will encourage substances to attack them Combustion - make useful fuels - especially the lower members of the series - react with oxygen in an exothermic reaction BUT Handy tip complete combustion CH 4(g) + 2 O 2(g) incomplete combustion CH 4(g) + 1½O 2(g) ——> CO 2(g) + 2 H 2 O(l) ——> CO(g) 2 H 2 O(l) + the greater the number of carbon atoms, the more energy produced the greater the amount of oxygen needed for complete combustion. When balancing equations involving complete combustion, remember. . . every carbon in the original hydrocarbon gives one carbon dioxide and every two hydrogen atoms gives a water molecule. Put the numbers into the equation, count up the O’s and H’s on the RHS of the equation then balance the oxygen molecules on the LHS.

POLLUTION Processes involving combustion give rise to a variety of pollutants. . . power stations internal combustion engines Removal SO 2 CO and NOx SO 2 emissions produce acid rain CO, NOx and unburnt hydrocarbons react effluent gases with a suitable compound (e. g. Ca. O) pass exhaust gases through a catalytic converter Catalytic converters In the catalytic converter. . . CO is converted to CO 2 NOx are converted to N 2 Unburnt hydrocarbons are converted to CO 2 and H 2 O e. g. 2 NO + 2 CO ———> N 2 + 2 CO 2 • catalysts are made of finely divided rare metals Rh, Pd, Pt • leaded petrol must not pass through the catalyst as the lead deposits on the catalyst’s surface and “poisons” it, thus blocking sites for reactions to take place.

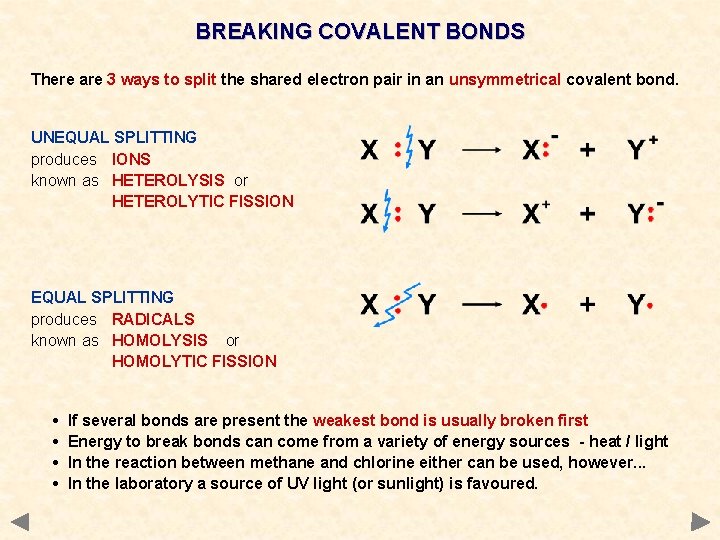
BREAKING COVALENT BONDS There are 3 ways to split the shared electron pair in an unsymmetrical covalent bond. UNEQUAL SPLITTING produces IONS known as HETEROLYSIS or HETEROLYTIC FISSION EQUAL SPLITTING produces RADICALS known as HOMOLYSIS or HOMOLYTIC FISSION • • If several bonds are present the weakest bond is usually broken first Energy to break bonds can come from a variety of energy sources - heat / light In the reaction between methane and chlorine either can be used, however. . . In the laboratory a source of UV light (or sunlight) is favoured.

FREE RADICALS TYPICAL PROPERTIES • reactive species (atoms or groups) which possess an unpaired electron • their reactivity is due to them wanting to pair up the single electron • formed by homolytic fission (homolysis) of covalent bonds • formed during the reaction between chlorine and methane • formed during thermal cracking • involved in the reactions taking place in the ozone layer

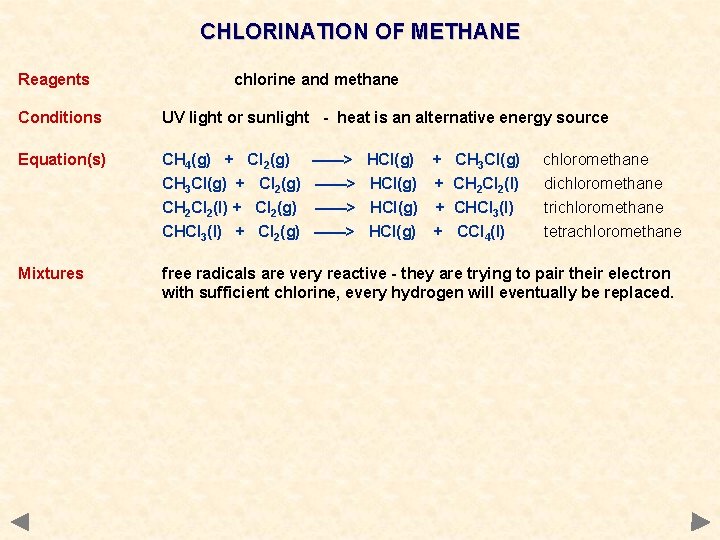
CHLORINATION OF METHANE Reagents chlorine and methane Conditions UV light or sunlight - heat is an alternative energy source Equation(s) CH 4(g) + Cl 2(g) CH 3 Cl(g) + Cl 2(g) CH 2 Cl 2(l) + Cl 2(g) CHCl 3(l) + Cl 2(g) Mixtures free radicals are very reactive - they are trying to pair their electron with sufficient chlorine, every hydrogen will eventually be replaced. ——> ——> HCl(g) + CH 3 Cl(g) HCl(g) + CH 2 Cl 2(l) HCl(g) + CHCl 3(l) HCl(g) + CCl 4(l) chloromethane dichloromethane trichloromethane tetrachloromethane

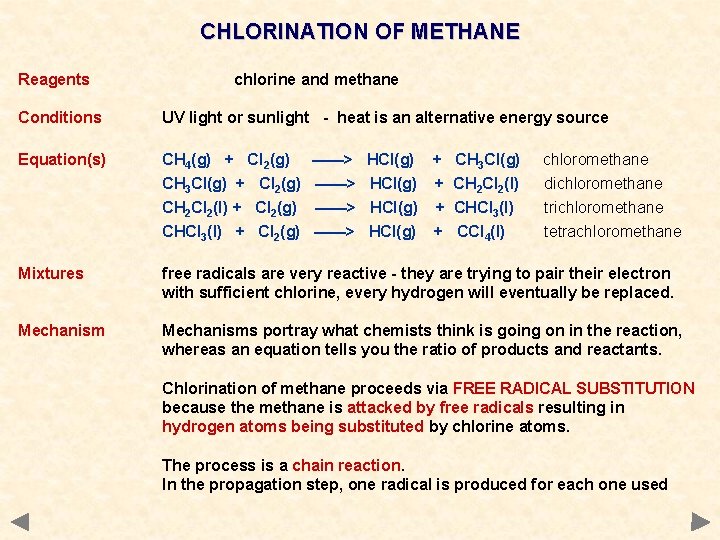
CHLORINATION OF METHANE Reagents chlorine and methane Conditions UV light or sunlight - heat is an alternative energy source Equation(s) CH 4(g) + Cl 2(g) CH 3 Cl(g) + Cl 2(g) CH 2 Cl 2(l) + Cl 2(g) CHCl 3(l) + Cl 2(g) Mixtures free radicals are very reactive - they are trying to pair their electron with sufficient chlorine, every hydrogen will eventually be replaced. Mechanisms portray what chemists think is going on in the reaction, whereas an equation tells you the ratio of products and reactants. ——> ——> HCl(g) + CH 3 Cl(g) HCl(g) + CH 2 Cl 2(l) HCl(g) + CHCl 3(l) HCl(g) + CCl 4(l) chloromethane dichloromethane trichloromethane tetrachloromethane Chlorination of methane proceeds via FREE RADICAL SUBSTITUTION because the methane is attacked by free radicals resulting in hydrogen atoms being substituted by chlorine atoms. The process is a chain reaction. In the propagation step, one radical is produced for each one used

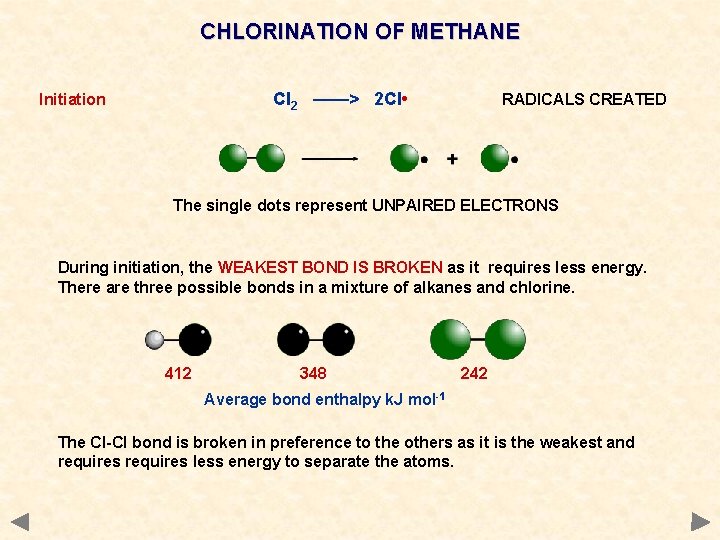
CHLORINATION OF METHANE Cl 2 ——> 2 Cl • Initiation RADICALS CREATED The single dots represent UNPAIRED ELECTRONS During initiation, the WEAKEST BOND IS BROKEN as it requires less energy. There are three possible bonds in a mixture of alkanes and chlorine. 412 348 242 Average bond enthalpy k. J mol-1 The Cl-Cl bond is broken in preference to the others as it is the weakest and requires less energy to separate the atoms.

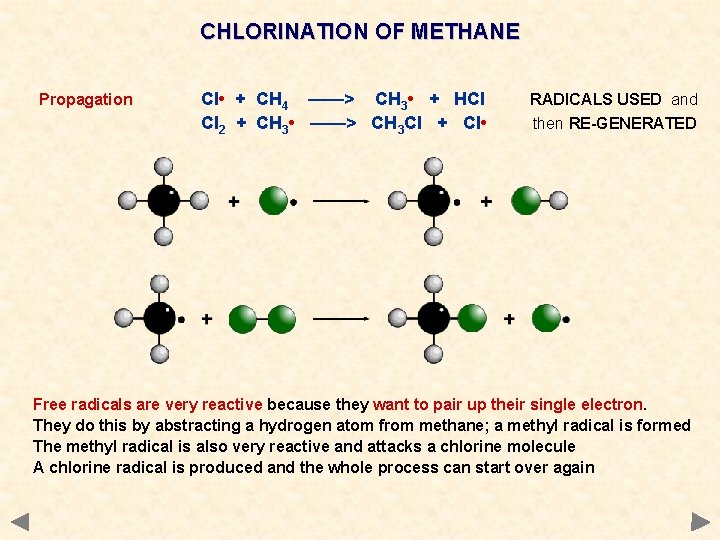
CHLORINATION OF METHANE Propagation Cl • + CH 4 ——> CH 3 • + HCl Cl 2 + CH 3 • ——> CH 3 Cl + Cl • RADICALS USED and then RE-GENERATED Free radicals are very reactive because they want to pair up their single electron. They do this by abstracting a hydrogen atom from methane; a methyl radical is formed The methyl radical is also very reactive and attacks a chlorine molecule A chlorine radical is produced and the whole process can start over again

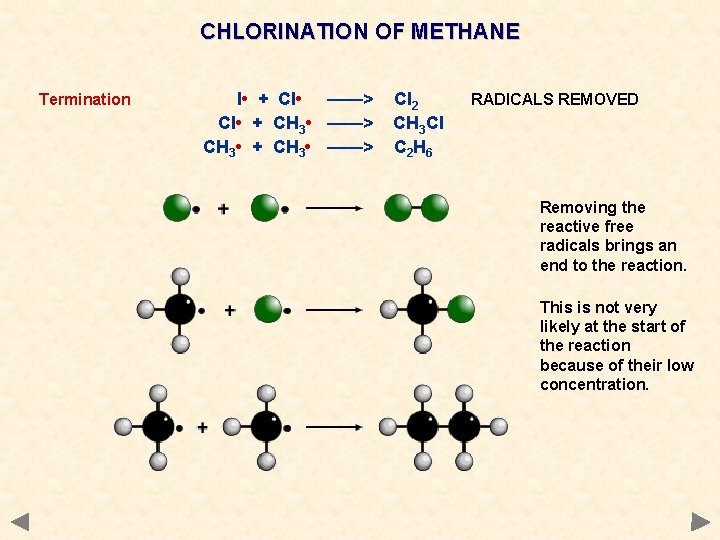
CHLORINATION OF METHANE Termination l • + Cl • ——> Cl • + CH 3 • ——> CH 3 • + CH 3 • ——> Cl 2 CH 3 Cl C 2 H 6 RADICALS REMOVED Removing the reactive free radicals brings an end to the reaction. This is not very likely at the start of the reaction because of their low concentration.

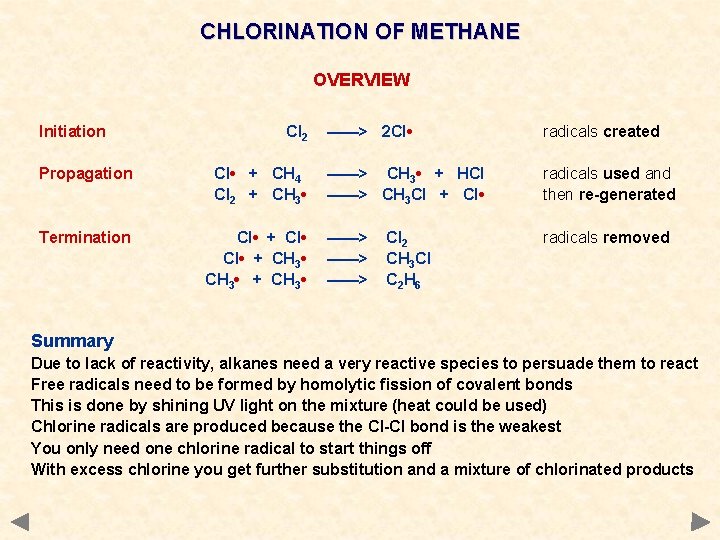
CHLORINATION OF METHANE OVERVIEW Initiation Cl 2 Propagation Cl • + CH 4 Cl 2 + CH 3 • Termination Cl • + CH 3 • + CH 3 • ——> 2 Cl • radicals created ——> CH 3 • + HCl ——> CH 3 Cl + Cl • radicals used and then re-generated ——> ——> radicals removed Cl 2 CH 3 Cl C 2 H 6 Summary Due to lack of reactivity, alkanes need a very reactive species to persuade them to react Free radicals need to be formed by homolytic fission of covalent bonds This is done by shining UV light on the mixture (heat could be used) Chlorine radicals are produced because the Cl-Cl bond is the weakest You only need one chlorine radical to start things off With excess chlorine you get further substitution and a mixture of chlorinated products

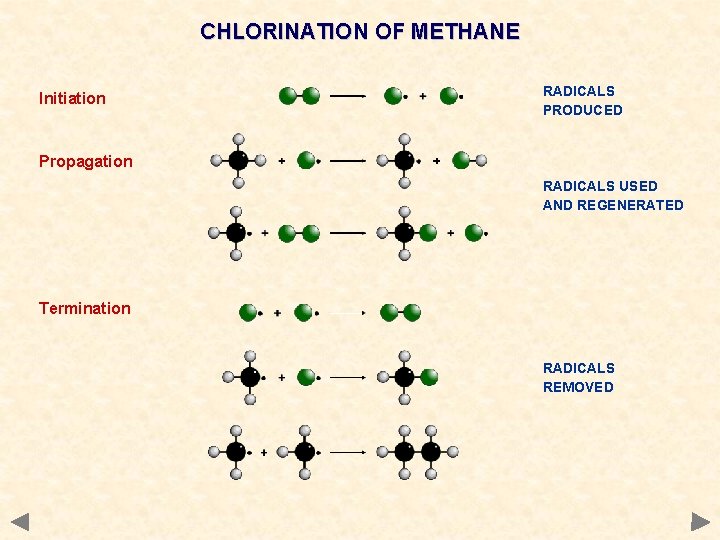
CHLORINATION OF METHANE Initiation RADICALS PRODUCED Propagation RADICALS USED AND REGENERATED Termination RADICALS REMOVED

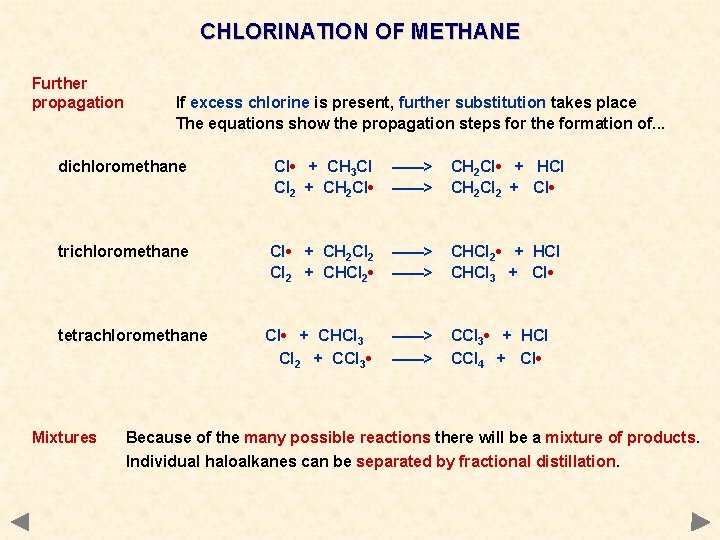
CHLORINATION OF METHANE Further propagation If excess chlorine is present, further substitution takes place The equations show the propagation steps for the formation of. . . dichloromethane Cl • + CH 3 Cl Cl 2 + CH 2 Cl • ——> CH 2 Cl • + HCl CH 2 Cl 2 + Cl • trichloromethane Cl • + CH 2 Cl 2 + CHCl 2 • ——> CHCl 2 • + HCl CHCl 3 + Cl • tetrachloromethane Cl • + CHCl 3 Cl 2 + CCl 3 • ——> CCl 3 • + HCl CCl 4 + Cl • Mixtures Because of the many possible reactions there will be a mixture of products. Individual haloalkanes can be separated by fractional distillation.

CRACKING Involves the breaking of C-C bonds in alkanes Converts heavy fractions into higher value products THERMAL CATALYTIC proceeds via a free radical mechanism proceeds via a carbocation (carbonium ion) mechanism THERMAL HIGH PRESSURE. . . 7000 k. Pa HIGH TEMPERATURE. . . 400°C to 900°C FREE RADICAL MECHANISM HOMOLYTIC FISSION PRODUCES MOSTLY ALKENES. . . e. g. ETHENE for making polymers and ethanol PRODUCES HYDROGEN. . . used in the Haber Process and in margarine manufacture Bonds can be broken anywhere in the molecule by C-C bond fission or C-H bond fission

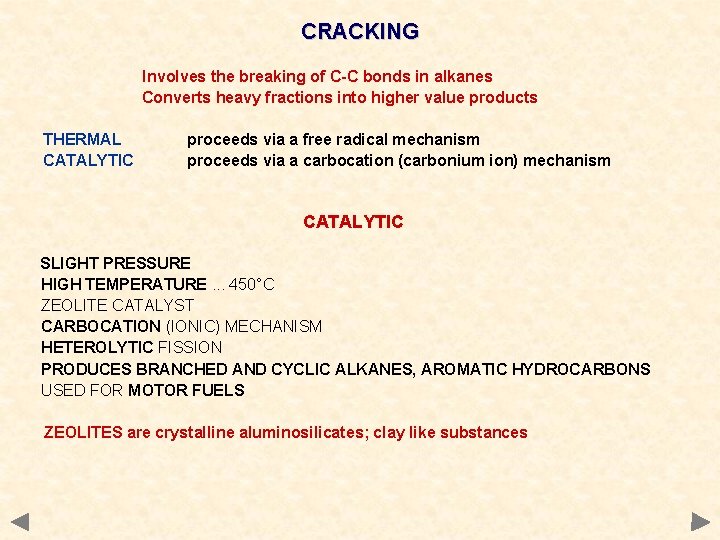
CRACKING Involves the breaking of C-C bonds in alkanes Converts heavy fractions into higher value products THERMAL CATALYTIC proceeds via a free radical mechanism proceeds via a carbocation (carbonium ion) mechanism CATALYTIC SLIGHT PRESSURE HIGH TEMPERATURE. . . 450°C ZEOLITE CATALYST CARBOCATION (IONIC) MECHANISM HETEROLYTIC FISSION PRODUCES BRANCHED AND CYCLIC ALKANES, AROMATIC HYDROCARBONS USED FOR MOTOR FUELS ZEOLITES are crystalline aluminosilicates; clay like substances

REVISION CHECK What should you be able to do? Recall and explain the physical properties of alkanes Recall the use of alkanes as fuels Recall and explain the different ways to break a covalent bond Write balanced equations representing combustion and chlorination Understand the conditions and mechanism of free radical substitution Recall the conditions and products from thermal and catalytic cracking CAN YOU DO ALL OF THESE? YES NO

You need to go over the relevant topic(s) again Click on the button to return to the menu

WELL DONE! Try some past paper questions

AN INTRODUCTION TO THE CHEMISTRY OF ALKANES THE END © 2003 JONATHAN HOPTON & KNOCKHARDY PUBLISHING
 Knockhardy notes
Knockhardy notes Knockhardy
Knockhardy Knockhardy
Knockhardy Knockhardy chemistry
Knockhardy chemistry Knockhardy science
Knockhardy science Knockhardy
Knockhardy Knockhardy chemistry a level
Knockhardy chemistry a level Knockhardy chemistry
Knockhardy chemistry Knockhardy notes
Knockhardy notes Types of halogenoalkanes
Types of halogenoalkanes Knockhardy
Knockhardy Alkanes solubility
Alkanes solubility Isomers of hexane structure
Isomers of hexane structure General molecular formula of alkene
General molecular formula of alkene Uses of alkanes
Uses of alkanes Alkanes list
Alkanes list Uses of alkanes
Uses of alkanes Alkanes alkenes alkynes
Alkanes alkenes alkynes Alkanes are also called
Alkanes are also called Terminal alkyne ir spectrum
Terminal alkyne ir spectrum Combustion of alkynes
Combustion of alkynes Alkanes combustion
Alkanes combustion Alkanes def
Alkanes def Alkylation of alkanes
Alkylation of alkanes Viscosity of alkanes
Viscosity of alkanes Cyclopentane uses
Cyclopentane uses Iupac stands for
Iupac stands for Stability of chair and boat conformation
Stability of chair and boat conformation Bromine water test
Bromine water test