Amides Nomenclature of amides IUPAC system for naming

![Reduction Amides are reduced to amines CH 3 CONH 2 + 4[H] Li. Al. Reduction Amides are reduced to amines CH 3 CONH 2 + 4[H] Li. Al.](https://slidetodoc.com/presentation_image_h2/562106a11d2bab00131baedd772ccb65/image-10.jpg)
- Slides: 10

Amides

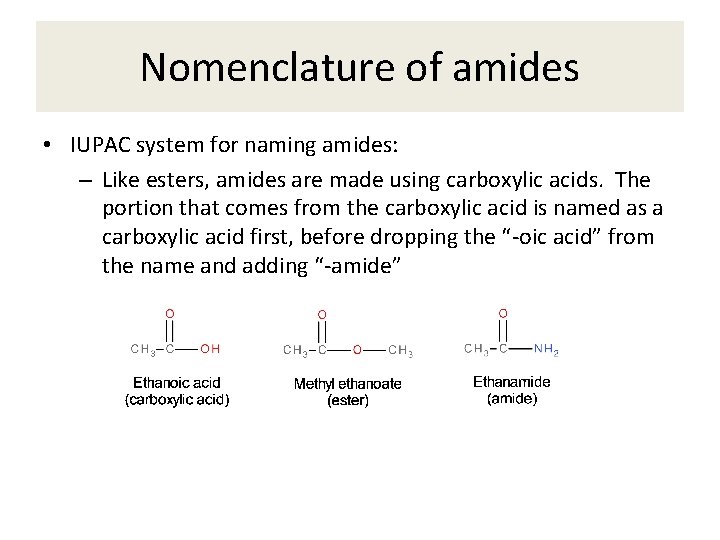
Nomenclature of amides • IUPAC system for naming amides: – Like esters, amides are made using carboxylic acids. The portion that comes from the carboxylic acid is named as a carboxylic acid first, before dropping the “-oic acid” from the name and adding “-amide”

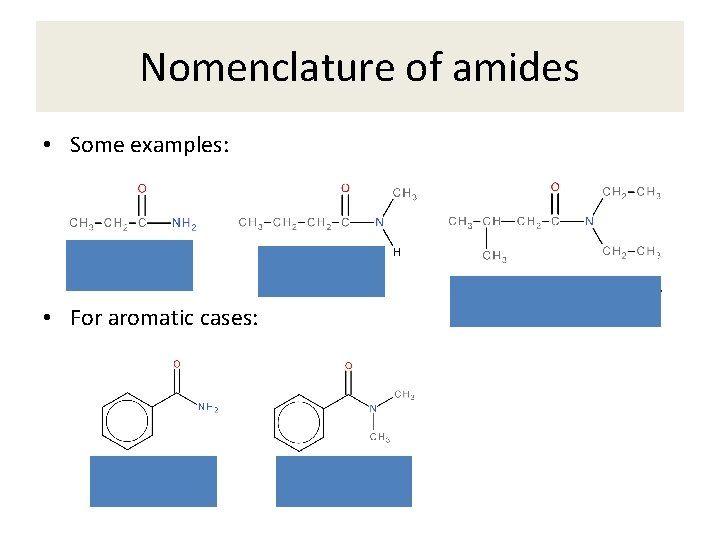
Nomenclature of amides • Some examples: • For aromatic cases:

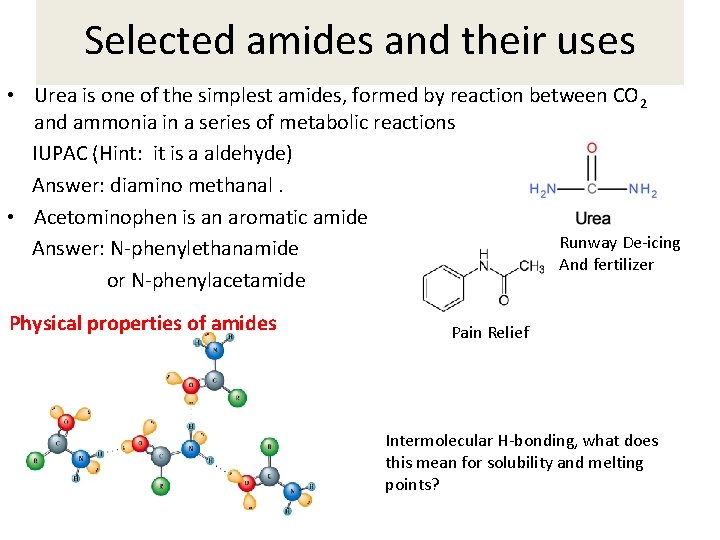
Selected amides and their uses • Urea is one of the simplest amides, formed by reaction between CO 2 and ammonia in a series of metabolic reactions IUPAC (Hint: it is a aldehyde) Answer: diamino methanal. • Acetominophen is an aromatic amide Runway De-icing Answer: N-phenylethanamide And fertilizer or N-phenylacetamide Physical properties of amides Pain Relief Intermolecular H-bonding, what does this mean for solubility and melting points?

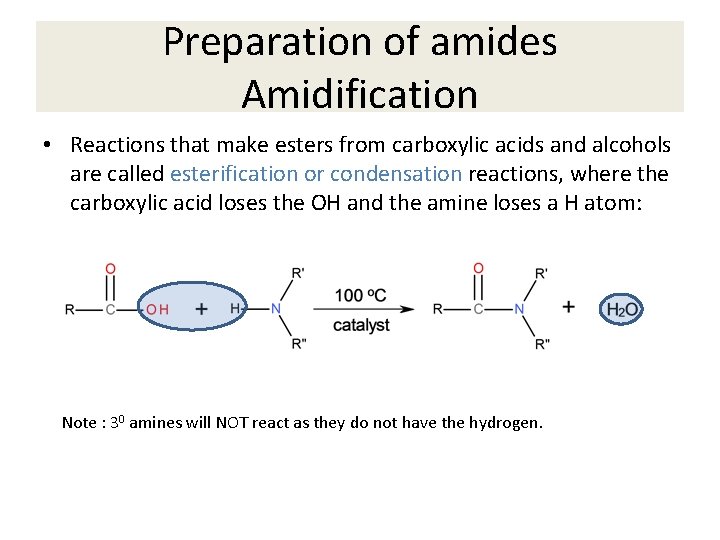
Preparation of amides Amidification • Reactions that make esters from carboxylic acids and alcohols are called esterification or condensation reactions, where the carboxylic acid loses the OH and the amine loses a H atom: Note : 30 amines will NOT react as they do not have the hydrogen.

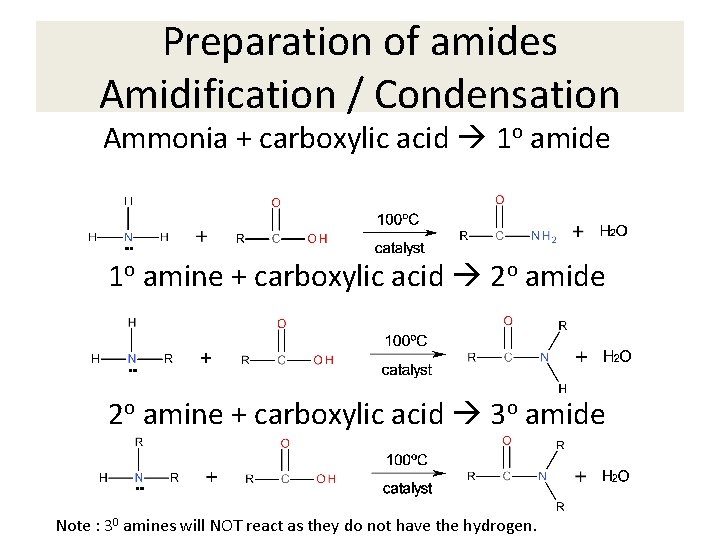
Preparation of amides Amidification / Condensation Ammonia + carboxylic acid 1 o amide 1 o amine + carboxylic acid 2 o amide 2 o amine + carboxylic acid 3 o amide Note : 30 amines will NOT react as they do not have the hydrogen.

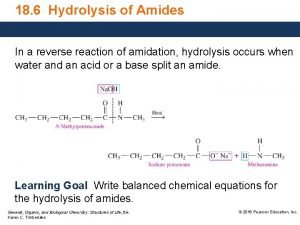
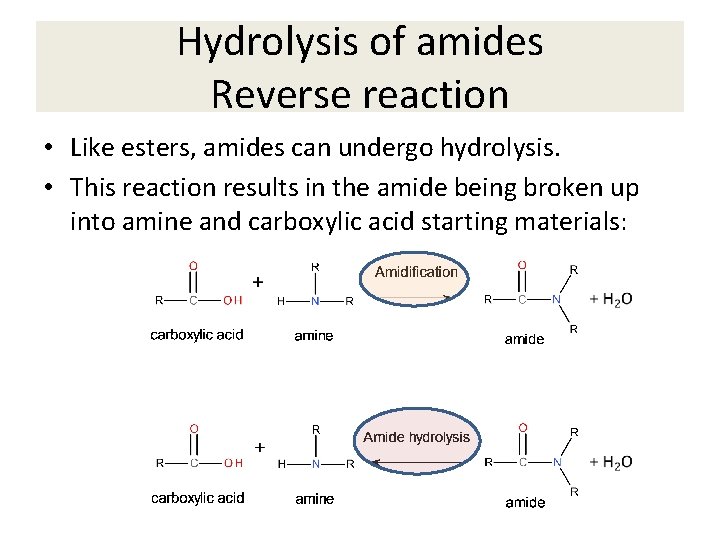
Hydrolysis of amides Reverse reaction • Like esters, amides can undergo hydrolysis. • This reaction results in the amide being broken up into amine and carboxylic acid starting materials:

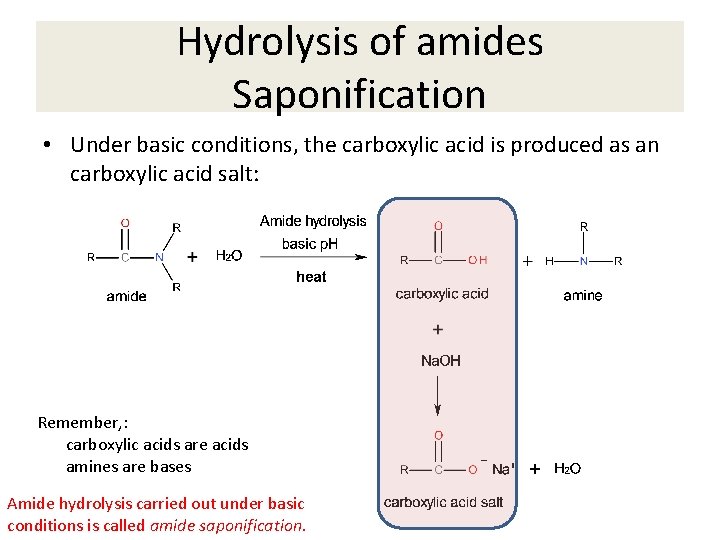
Hydrolysis of amides Saponification • Under basic conditions, the carboxylic acid is produced as an carboxylic acid salt: Remember, : carboxylic acids are acids amines are bases Amide hydrolysis carried out under basic conditions is called amide saponification.

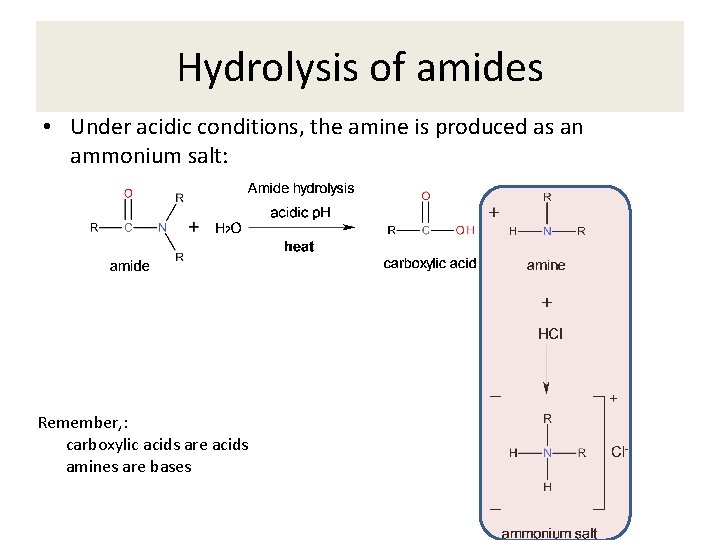
Hydrolysis of amides • Under acidic conditions, the amine is produced as an ammonium salt: Remember, : carboxylic acids are acids amines are bases
![Reduction Amides are reduced to amines CH 3 CONH 2 4H Li Al Reduction Amides are reduced to amines CH 3 CONH 2 + 4[H] Li. Al.](https://slidetodoc.com/presentation_image_h2/562106a11d2bab00131baedd772ccb65/image-10.jpg)
Reduction Amides are reduced to amines CH 3 CONH 2 + 4[H] Li. Al. H 4 CH 3 CH 2 NH 2 + H 2 O