Alcohols IUPAC Nomenclature of Alcohols Nomenclature The longest























- Slides: 31

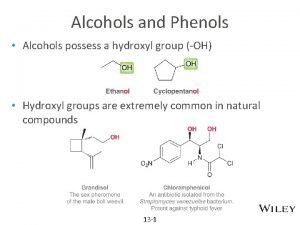
Alcohols

IUPAC Nomenclature of Alcohols

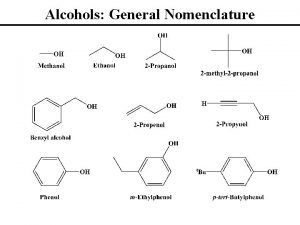
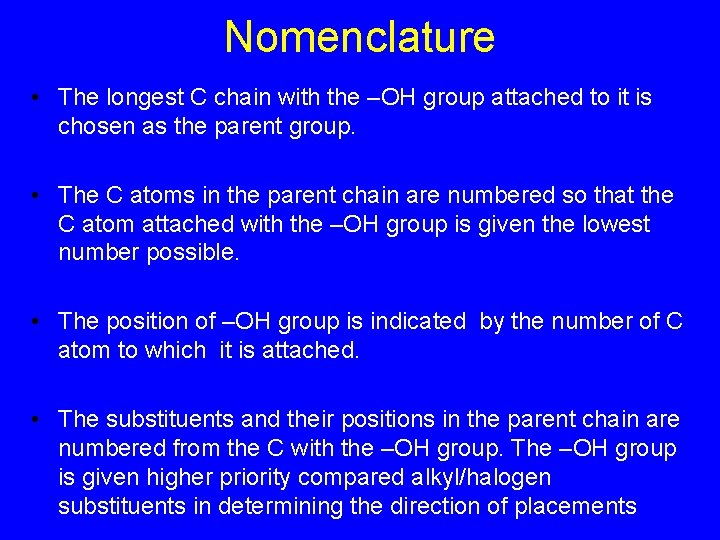
Nomenclature • The longest C chain with the –OH group attached to it is chosen as the parent group. • The C atoms in the parent chain are numbered so that the C atom attached with the –OH group is given the lowest number possible. • The position of –OH group is indicated by the number of C atom to which it is attached. • The substituents and their positions in the parent chain are numbered from the C with the –OH group. The –OH group is given higher priority compared alkyl/halogen substituents in determining the direction of placements

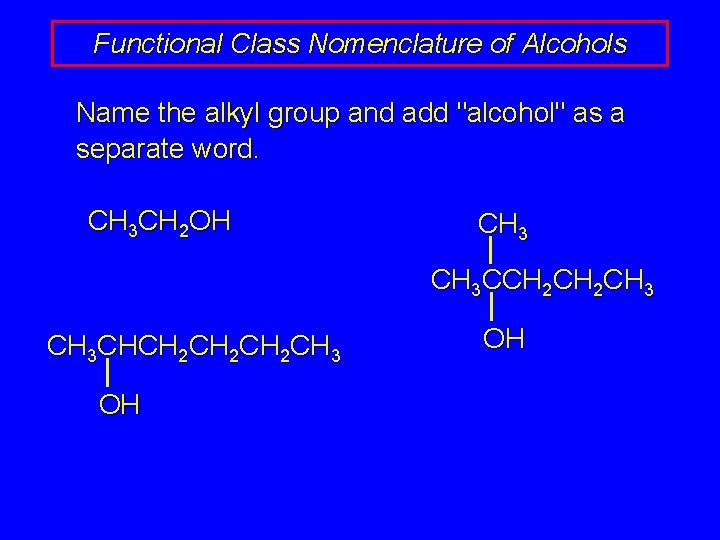
Functional Class Nomenclature of Alcohols Name the alkyl group and add "alcohol" as a separate word. CH 3 CH 2 OH CH 3 CCH 2 CH 3 CHCH 2 CH 2 CH 3 OH OH

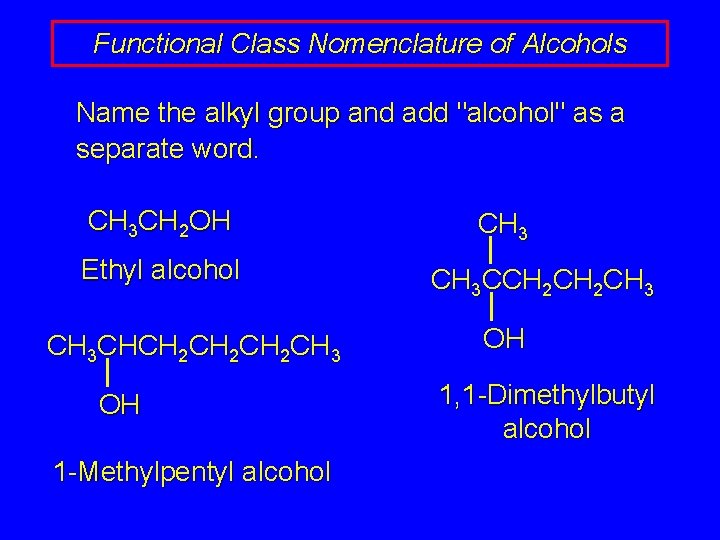
Functional Class Nomenclature of Alcohols Name the alkyl group and add "alcohol" as a separate word. CH 3 CH 2 OH Ethyl alcohol CH 3 CHCH 2 CH 2 CH 3 OH 1 -Methylpentyl alcohol CH 3 CCH 2 CH 3 OH 1, 1 -Dimethylbutyl alcohol

Classes of Alcohols

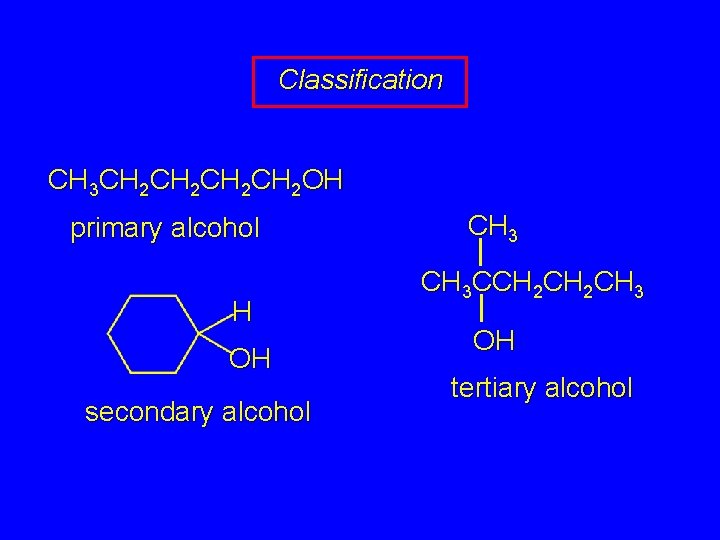
Classification Alcohols and alkyl halides are classified as primary secondary tertiary according to their "degree of substitution. " Degree of substitution is determined by counting the number of carbon atoms directly attached to the carbon that bears the halogen or hydroxyl group.

Classification CH 3 CH 2 CH 2 OH primary alcohol H OH secondary alcohol CH 3 CCH 2 CH 3 OH tertiary alcohol

Number of hydroxyl compound • Hydroxy compound that have only 1 OH group: monohydric alcohols. – Methanol, 2 -propanol • Have 2 –OH group: dihydric alcohols / diols. – 1, 2 -ethanediol, 1, 3 -propanediol. • Have 3 –OH group: trihydric alcohols / triols. – 1, 2, 3 -propanetriol.

Physical Properties of Alcohols

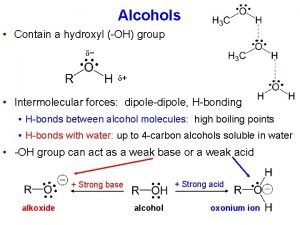
Boiling Points § Higher than other organic compounds with equivalent relative molecular mass. § Formation of hydrogen bond between –OH groups in alcohol molecule. § Boiling point increases as Molecular Mass of alcohol increase since the van der Waals forces of attraction increases with molecular size.

Boiling Points § Boiling point of branched chain alcohol is lower than straight chain, with same Molecular mass. § Small surface area, hence weaker van der Waals forces. § 3° alcohol < 2° alcohol < 1° alcohol boiling point increases

Solubility in Water § Lower members of alcohols are soluble in water; § Formation of H bond between water & alcohol. § Solubility in water decreases significantly: § Size of alkyl group, R § R is non-polar § Bigger influence when number of C (hence size) increases. § Order of solubility in water; § 3° alcohol < 2° alcohol < 1° alcohol solubility increases

• Due to stearic factor as alkyl, -R groups hinder the formation of H-bonds between the –OH groups and water molecules. • Polyhydric alcohols are more soluble in water than monohydric alcohols. • Triol > diol > monohydric alcohols Solubility in water decreases this is because the more –OH groups present in a molecule, the more hydrogen bonds are formed with water.

Reactions of Hydroxyl Compounds

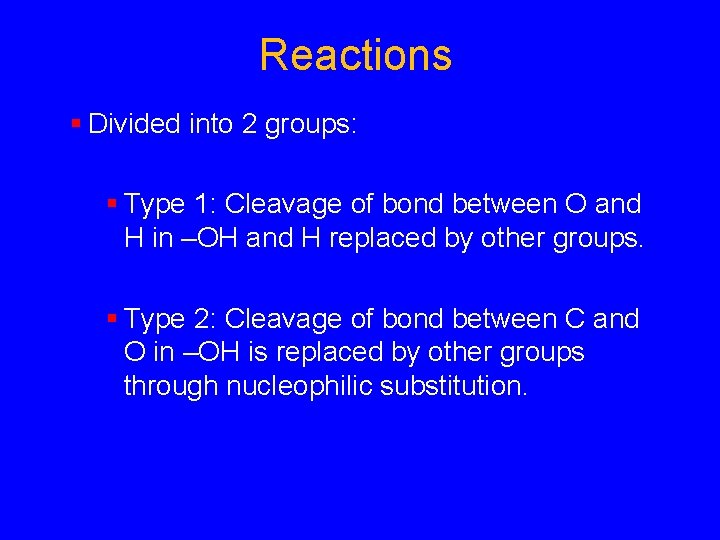
Reactions § Divided into 2 groups: § Type 1: Cleavage of bond between O and H in –OH and H replaced by other groups. § Type 2: Cleavage of bond between C and O in –OH is replaced by other groups through nucleophilic substitution.

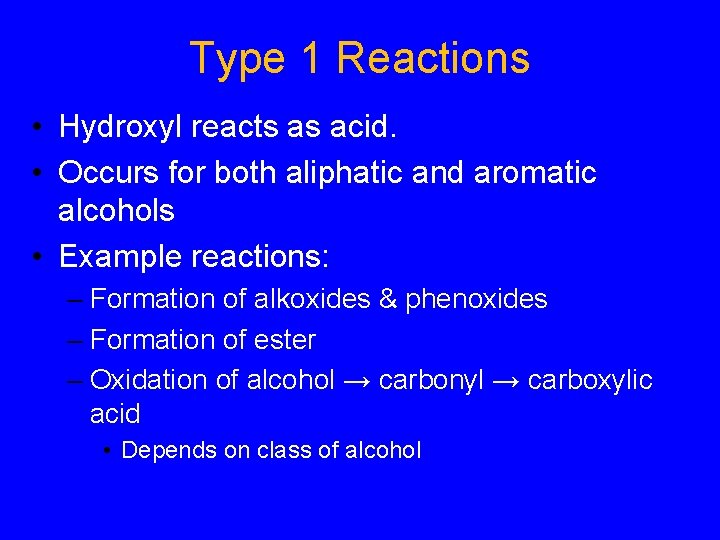
Type 1 Reactions • Hydroxyl reacts as acid. • Occurs for both aliphatic and aromatic alcohols • Example reactions: – Formation of alkoxides & phenoxides – Formation of ester – Oxidation of alcohol → carbonyl → carboxylic acid • Depends on class of alcohol

Type 2 Reactions • Hydroxyl react as base. • Occurs in aliphatic alcohols only. • Example reactions: – Rxn with hydrogen halides, phosphorus halide / thionyl chloride. – Dehydration → alkene / ethers.

T 1: Formation of alkoxides & Phenoxides • Alcohol & Phenol react with electropositive metals (Na/K) to form salt known as alkoxides/phenoxides & H 2 gas.

Application • Qualitative test for the presence of –OH group. – H 2 gas released when Na? K react with compound X. X could be alcohol/carboxylic acid • Quantitative test for the number of –OH groups. • To generate H 2 gas that is newly formed to carry out reduction reactions.

T 1: Esterification • Aliphatic alcohols + carboxylic acid → ester + water. • Aromatic compound → no rxn. • Acylation: – Both aliphatic & aromatic + acyl chloride → ester.

T 1: Oxidation • Alcohol can be oxidised to form carbonyl compound and carboxylic acid – depend on class of alcohol. • Involves removing 2 H atoms. • Hot acidified potassium dichromate (VI) / potassium manganate (VII) used. • 1° alcohol → aldehyde → carboxylic acid. • 2° alcohol → ketone: stable toward oxidizing agent. • 3° alcohol → resistance toward oxidation.

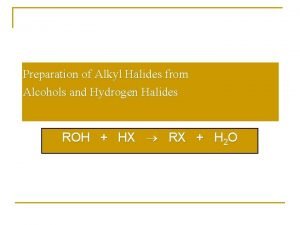
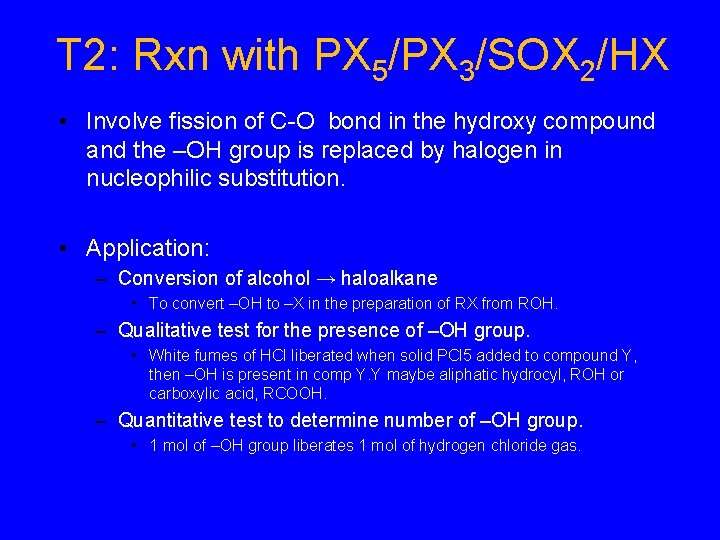
T 2: Rxn with PX 5/PX 3/SOX 2/HX • Involve fission of C-O bond in the hydroxy compound and the –OH group is replaced by halogen in nucleophilic substitution. • Application: – Conversion of alcohol → haloalkane • To convert –OH to –X in the preparation of RX from ROH. – Qualitative test for the presence of –OH group. • White fumes of HCl liberated when solid PCl 5 added to compound Y, then –OH is present in comp Y. Y maybe aliphatic hydrocyl, ROH or carboxylic acid, RCOOH. – Quantitative test to determine number of –OH group. • 1 mol of –OH group liberates 1 mol of hydrogen chloride gas.

• Application cont. : – In the rxn of thionyl chloride (sulfur dichloride oxide), SOCl 2 with alcohol, the chloroalkane produce can be easily isolated as the liquid as the rest of the by-products (SO 2 & HCl) are gases. – Alcohol react withconc. HCl / HBr to produce haloalkane. • Lucas Reagent: mixt of conc. HCl & Zn. Cl 2 • Distinguish class of alcohol, rate of reaction is different. – 1° alcohol: react very slowly, no cloudiness at room temperature. – 2° alcohol: react in 1 -5 min (solution turn cloudy after 5 min). – 3° alcohol: react almost instantaneously (immediate cloudiness)

T 2: Dehydration rxn • Two types of dehydration producing diff. product at diff. condition. – Intramolecular elimination of water. – Intermolecular elimination of water. • Intramolecular elimination of water from hydroxyl group & alpha H produce alkene. – α-H: H attached to C adjacent to –OH group. – By refluxing the alcohol with excess conc. H 2 SO 4 / H 3 PO 4 at temp. of 170 -180°C / heated with alumina. • Intermolecular elimination of water from two alcohol molecules to produce ether. – Conc. H 2 SO 4 and excess alcohol refluxed at temp. of 140°C.

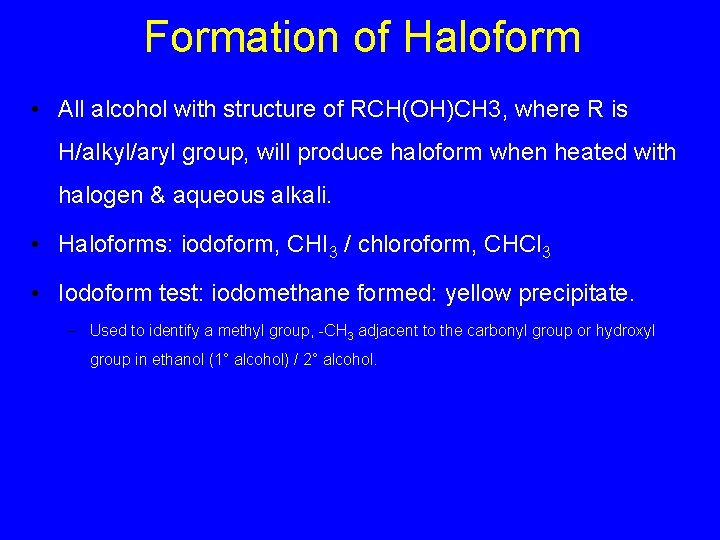
Formation of Haloform • All alcohol with structure of RCH(OH)CH 3, where R is H/alkyl/aryl group, will produce haloform when heated with halogen & aqueous alkali. • Haloforms: iodoform, CHI 3 / chloroform, CHCl 3 • Iodoform test: iodomethane formed: yellow precipitate. – Used to identify a methyl group, -CH 3 adjacent to the carbonyl group or hydroxyl group in ethanol (1° alcohol) / 2° alcohol.

Reactions of the Benzene Ring in Phenol

• Since –OH group in ortho- and para- directing, phenol undergo electrophilic substitution reactions in the 2 -(ortho) and 4 -(para) positions of benzene ring under mild conditions. • The electrophilic substitutions ofphenol include: – Halogenation with chlorine / bromine water. – Nitration with conc. Nitric acid – Friedel-Crafts alkylation & acylation.

Preparation of Hydroxyl Compound.

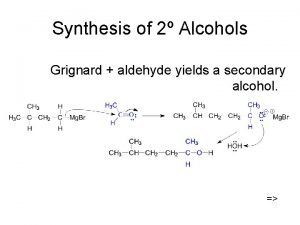
Preparation: Aliphatic Alcohol 1. Hyration of alkenes. 2. Hydrolysis of haloalkanes. 3. Reaction between Grignard reagents & carbonyl compounds. 4. Reduction of carbonyl compound. 5. Fermentation of carbohydrate.

Preparation: Phenol 1. Hydrolysis of chlorobenzene. 2. Cumene process 3. Hydroysis of diazonium salt
 Alcohols nomenclature
Alcohols nomenclature Naming alkyl halides
Naming alkyl halides Ethers naming
Ethers naming Diol oxidation
Diol oxidation Functional groups table class 10
Functional groups table class 10 Iupac nomenclature of cycloalkanes
Iupac nomenclature of cycloalkanes Carboxylic acid to ester
Carboxylic acid to ester Reactions of alcohols 2 chemsheets answers
Reactions of alcohols 2 chemsheets answers Names of sugar alcohols
Names of sugar alcohols Preparation of alcohol from grignard reagent
Preparation of alcohol from grignard reagent Naocl reaction with alcohol
Naocl reaction with alcohol Propan-1-ol oxidation
Propan-1-ol oxidation These are alcohols containing cppp nucleus?
These are alcohols containing cppp nucleus? Lucas test reagent
Lucas test reagent What does na2cr2o7 do
What does na2cr2o7 do Lucas reaction
Lucas reaction Secondary alcohols
Secondary alcohols Preparation of alkyl halides from alcohols
Preparation of alkyl halides from alcohols Acidity of alcohols
Acidity of alcohols Alcohols phenols thiols and ethers
Alcohols phenols thiols and ethers Oxidation of tertiary alcohol
Oxidation of tertiary alcohol Nomenclatura iupac
Nomenclatura iupac Nomenclatura iupac
Nomenclatura iupac Sodium hydroxide iupac id sodium oxidanide
Sodium hydroxide iupac id sodium oxidanide Iupac name for cycloalkane
Iupac name for cycloalkane Propiedades fisicas de las cetonas
Propiedades fisicas de las cetonas Grupo funcional nitrilo
Grupo funcional nitrilo Metoxipropano
Metoxipropano Nome iupac
Nome iupac Benennung organischer verbindungen übungen
Benennung organischer verbindungen übungen Butane condensed structural formula
Butane condensed structural formula Pcl
Pcl