Air and Air Pollution G Tyler Millers Living







- Slides: 20

Air and Air Pollution G. Tyler Miller’s Living in the Environment 13 th Edition Chapter 17 Dr. Richard Clements Chattanooga State Technical Community College

Key Concepts Ø Structure and composition of the atmosphere Ø Types and sources of outdoor air pollution Ø Types, formation, and effects of smog Ø Sources and effects of acid deposition Ø Effects of air pollution Ø Prevention and control of air pollution

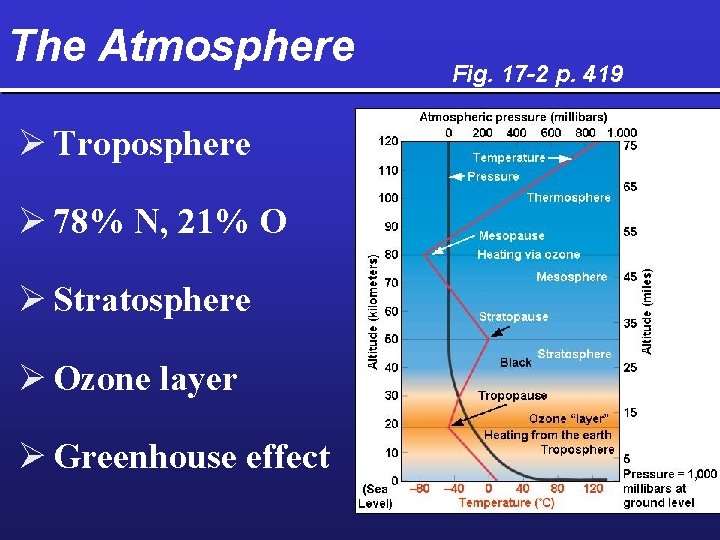
The Atmosphere Ø Troposphere Ø 78% N, 21% O Ø Stratosphere Ø Ozone layer Ø Greenhouse effect Fig. 17 -2 p. 419

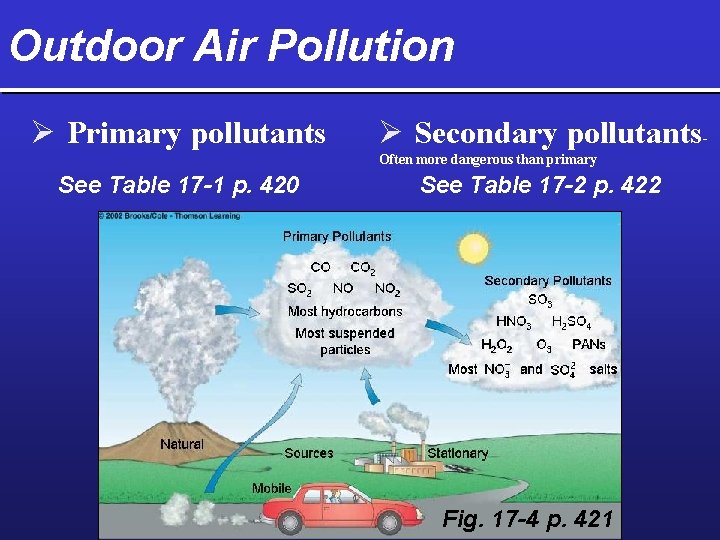
Outdoor Air Pollution Ø Primary pollutants Ø Secondary pollutants. Often more dangerous than primary See Table 17 -1 p. 420 See Table 17 -2 p. 422 Fig. 17 -4 p. 421

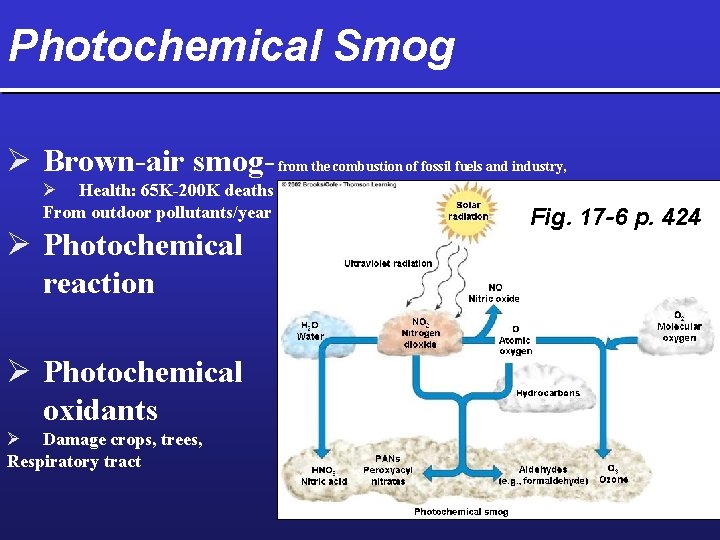
Photochemical Smog Ø Brown-air smog- from the combustion of fossil fuels and industry, Ø Health: 65 K-200 K deaths From outdoor pollutants/year Ø Photochemical reaction Ø Photochemical oxidants Ø Damage crops, trees, Respiratory tract Fig. 17 -6 p. 424

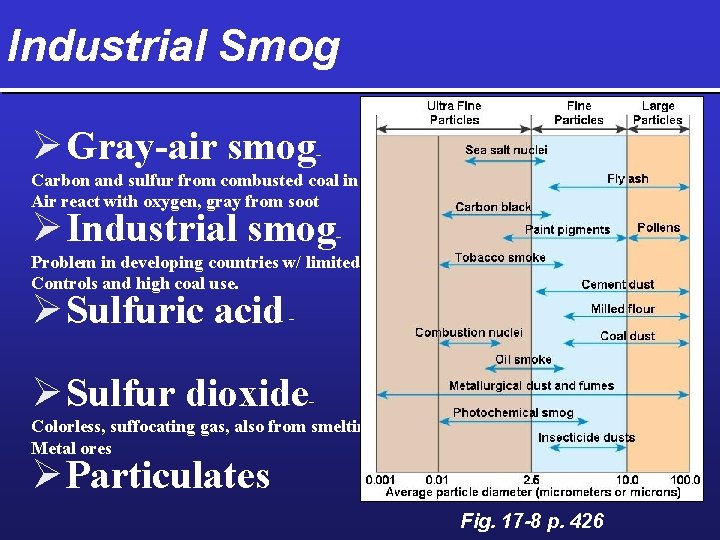
Industrial Smog Ø Gray-air smog. Carbon and sulfur from combusted coal in Air react with oxygen, gray from soot Ø Industrial smog- Problem in developing countries w/ limited Controls and high coal use. Ø Sulfuric acid - Ø Sulfur dioxide. Colorless, suffocating gas, also from smelting Metal ores Ø Particulates Fig. 17 -8 p. 426

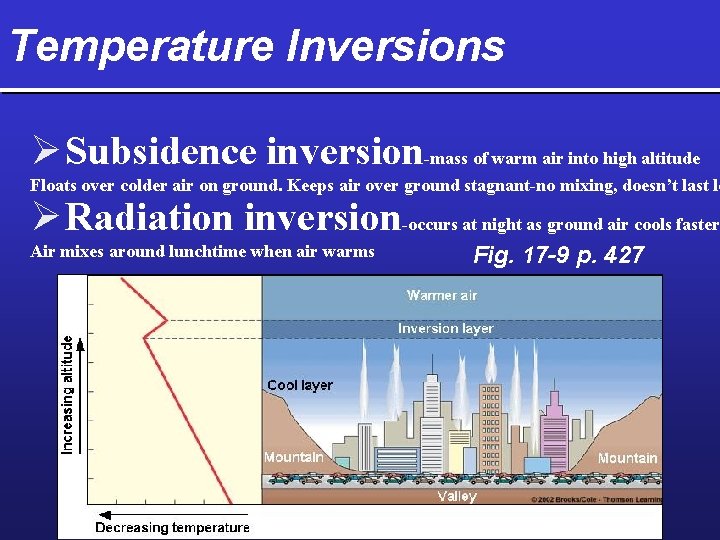
Temperature Inversions Ø Subsidence inversion-mass of warm air into high altitude Floats over colder air on ground. Keeps air over ground stagnant-no mixing, doesn’t last lo Ø Radiation inversion-occurs at night as ground air cools faster Air mixes around lunchtime when air warms Fig. 17 -9 p. 427

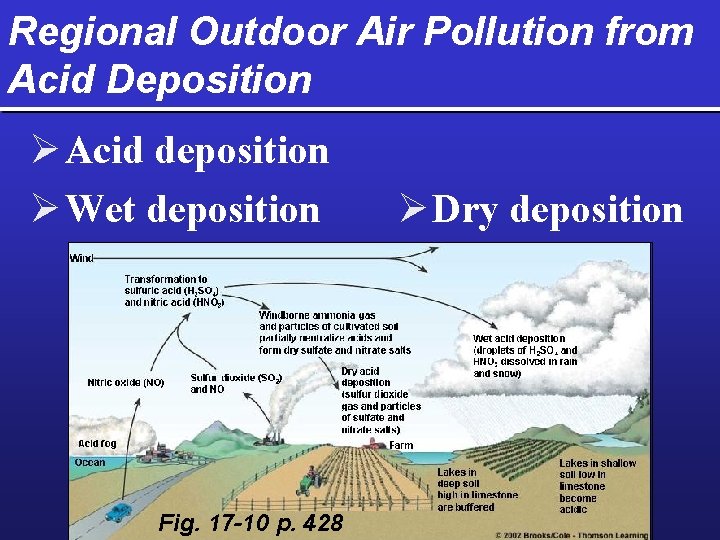
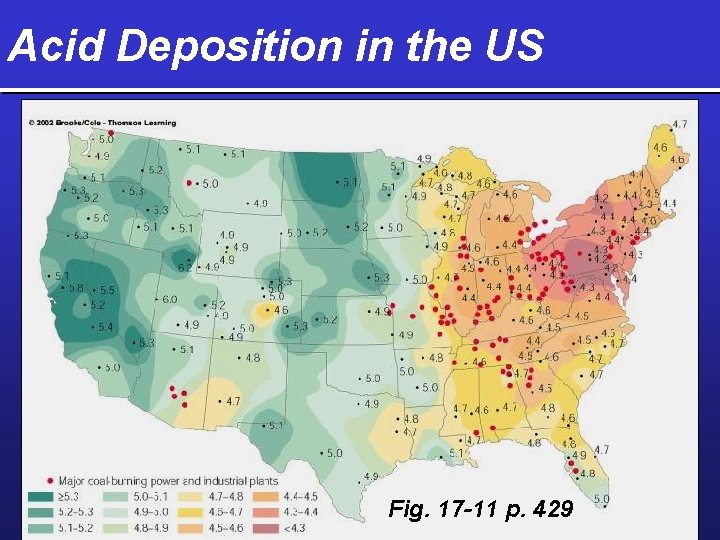
Regional Outdoor Air Pollution from Acid Deposition Ø Acid deposition Ø Wet deposition Fig. 17 -10 p. 428 Ø Dry deposition

Acid Deposition in the US Fig. 17 -11 p. 429

Acid Deposition and Humans Ø Respiratory diseases Ø Toxic metal leaching Ø Decreased visibility Ø Damage to structures, especially containing limestone Ø Decreased productivity and profitability of fisheries, forests, and farms

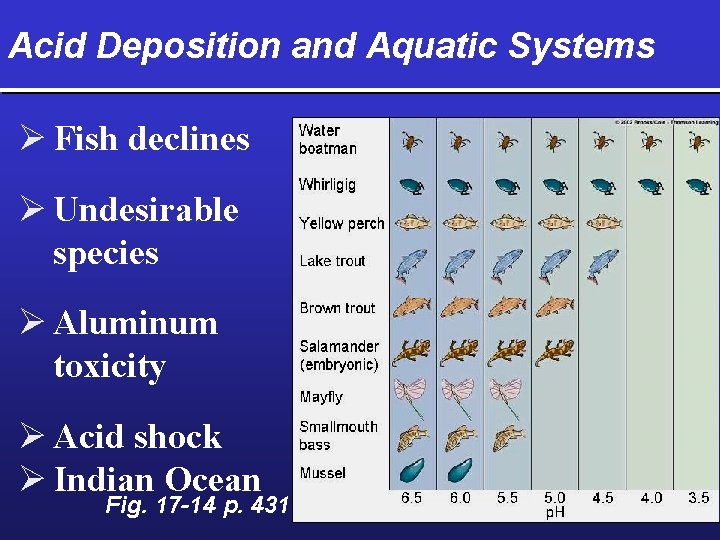
Acid Deposition and Aquatic Systems Ø Fish declines Ø Undesirable species Ø Aluminum toxicity Ø Acid shock Ø Indian Ocean Fig. 17 -14 p. 431

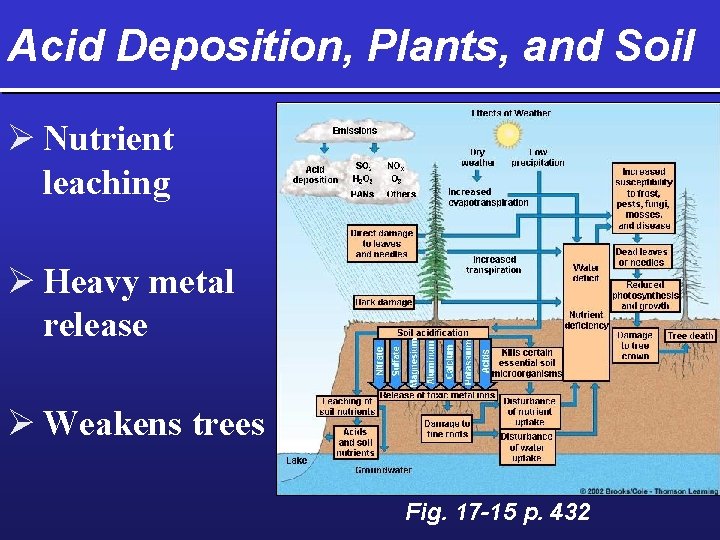
Acid Deposition, Plants, and Soil Ø Nutrient leaching Ø Heavy metal release Ø Weakens trees Fig. 17 -15 p. 432

Indoor Air Pollution/Sick Building Syndrome Fig. 17 -17 p. 434

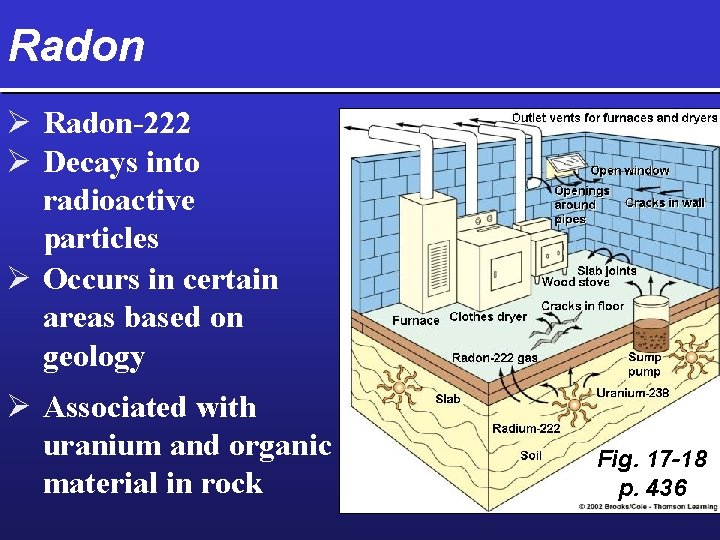
Radon Ø Radon-222 Ø Decays into radioactive particles Ø Occurs in certain areas based on geology Ø Associated with uranium and organic material in rock Fig. 17 -18 p. 436

Effects of Air Pollution on Living Organisms and Materials Ø Damage to mucous membranes Ø Respiratory diseases (see Fig. 17 -19 p. 438) Ø Damage to plant leaves and roots Ø Reduction in primary productivity Ø Deterioration of materials (See Table 17 -3 p. 440)

Solutions: Preventing and Reducing Air Pollution Ø Clean Air Act Ø Montreal Protocol-substances that deplete ozone 1987 Ø National Ambient Air Quality Standards (NAAQS)-6 principle pollutants- CO, Pb, NO 2, PM, O 3, SO 2 Ø Primary and secondary standards Ø Primary –human health, secondary –environmental health and damage Ø Output control vs. input control

Emission Reduction Fig. 17 -22 p. 441 Fig. 17 -23 a p. 442

Reducing Indoor Air Pollution Fig. 17 -25 p. 443

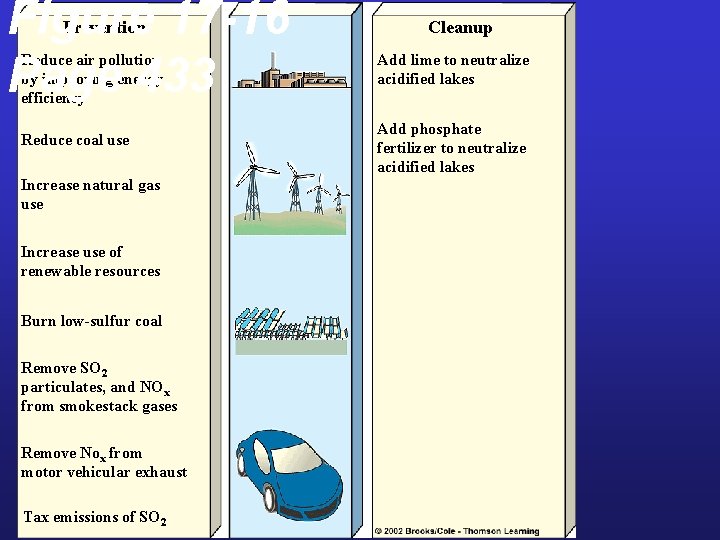
Figure 17 -16 Page 433 Prevention Reduce air pollution by improving energy efficiency Reduce coal use Increase natural gas use Increase use of renewable resources Burn low-sulfur coal Remove SO 2 particulates, and NOx from smokestack gases Remove Nox from motor vehicular exhaust Tax emissions of SO 2 Cleanup Add lime to neutralize acidified lakes Add phosphate fertilizer to neutralize acidified lakes

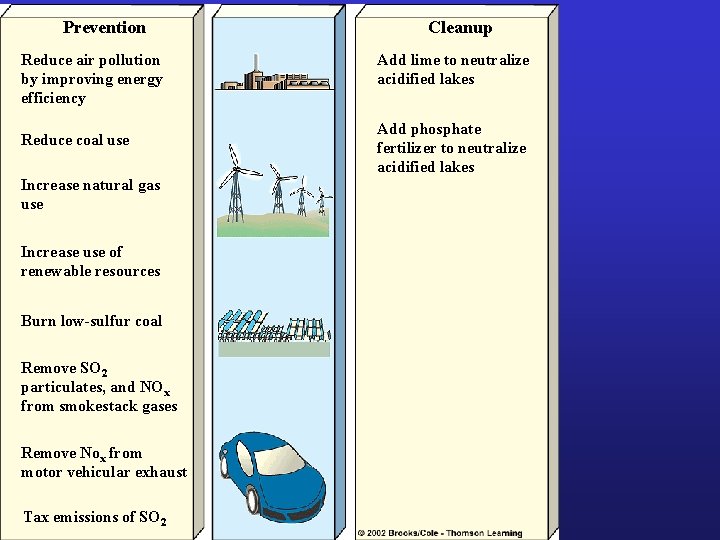
Prevention Reduce air pollution by improving energy efficiency Reduce coal use Increase natural gas use Increase use of renewable resources Burn low-sulfur coal Remove SO 2 particulates, and NOx from smokestack gases Remove Nox from motor vehicular exhaust Tax emissions of SO 2 Cleanup Add lime to neutralize acidified lakes Add phosphate fertilizer to neutralize acidified lakes
 Chapter 12 air section 1
Chapter 12 air section 1 Chapter 12 air section 1 what causes air pollution
Chapter 12 air section 1 what causes air pollution Millers right angle technique
Millers right angle technique Millers pyramid
Millers pyramid Millers liquefaction foci
Millers liquefaction foci Zones of dentinal caries
Zones of dentinal caries Arthur millers drama fra 1953
Arthur millers drama fra 1953 Glickman's concept
Glickman's concept Millers emerald
Millers emerald Beaded dentinal tubules
Beaded dentinal tubules Tuck one of robin hood's merry men
Tuck one of robin hood's merry men Ecosystem living and nonliving things
Ecosystem living and nonliving things Is a seed living or nonliving
Is a seed living or nonliving Living non living dead
Living non living dead The smallest living unit within the human body is
The smallest living unit within the human body is Effects of land pollution on human health
Effects of land pollution on human health Soil pollution images diagram
Soil pollution images diagram Aim or objectives of air pollution
Aim or objectives of air pollution What is primary and secondary air pollution
What is primary and secondary air pollution Ari rokeach
Ari rokeach Aim and objectives of air pollution
Aim and objectives of air pollution