Activity of Water Water Potential Plant Physiology UNI















- Slides: 42

Activity of Water = Water Potential Plant Physiology UNI Spring 2009

Overview • Physical part – Effect of temperature – Effect of pressure • Chemical part – Effects of solutes – Effects of hydrophilic solids • Combining to get water potential – Potential to do “work” (push, move)

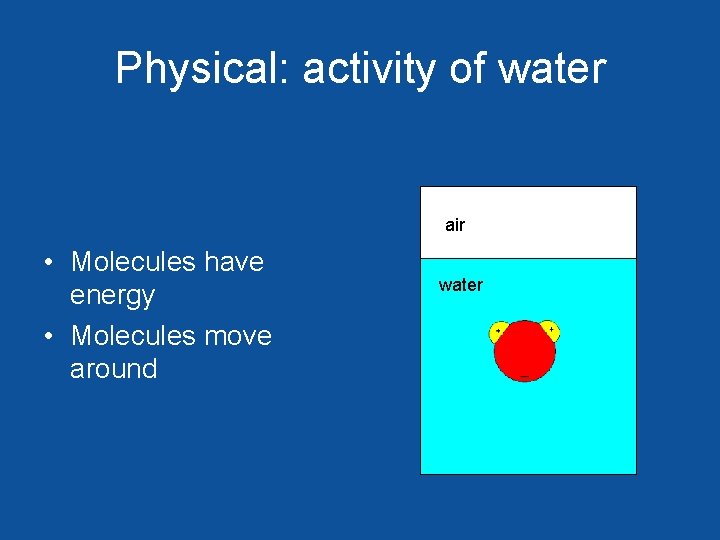
Physical: activity of water air • Molecules have energy • Molecules move around water

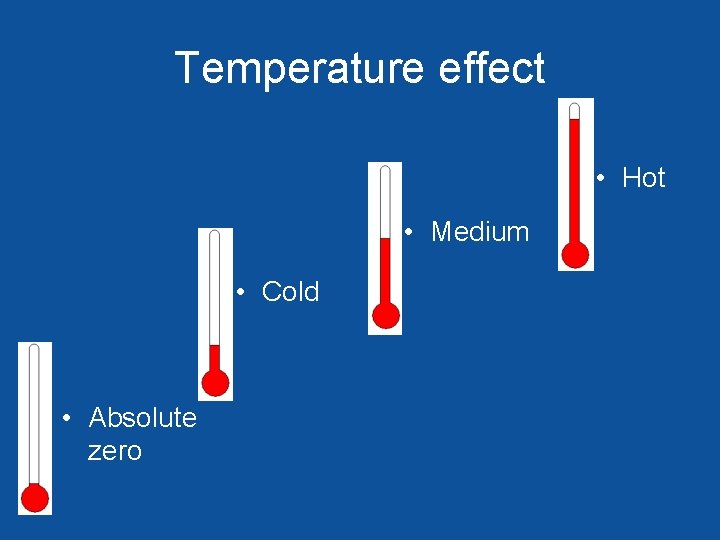
Temperature effect • Hot • Medium • Cold • Absolute zero

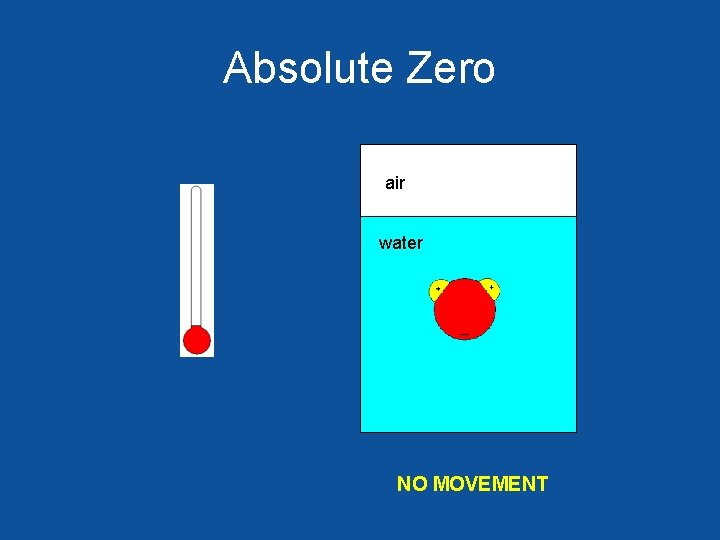
Absolute Zero air water NO MOVEMENT

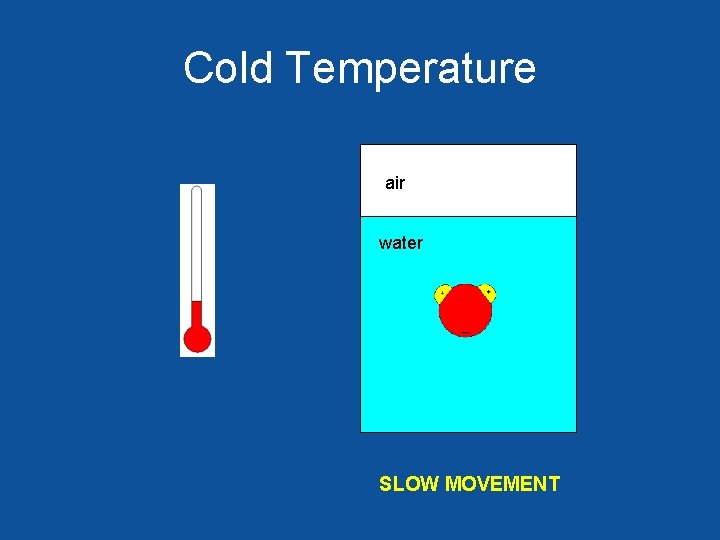
Cold Temperature air water SLOW MOVEMENT

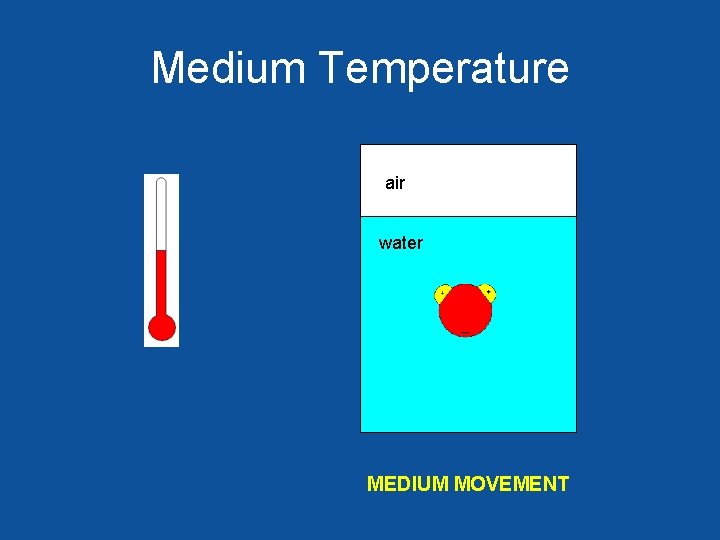
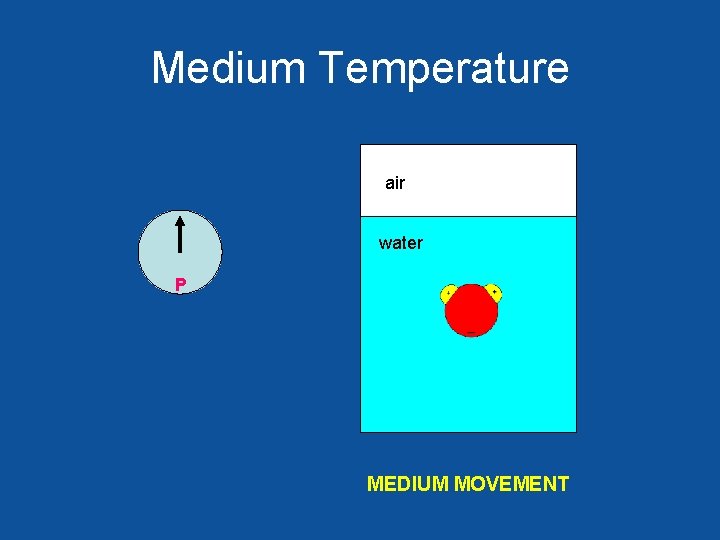
Medium Temperature air water MEDIUM MOVEMENT

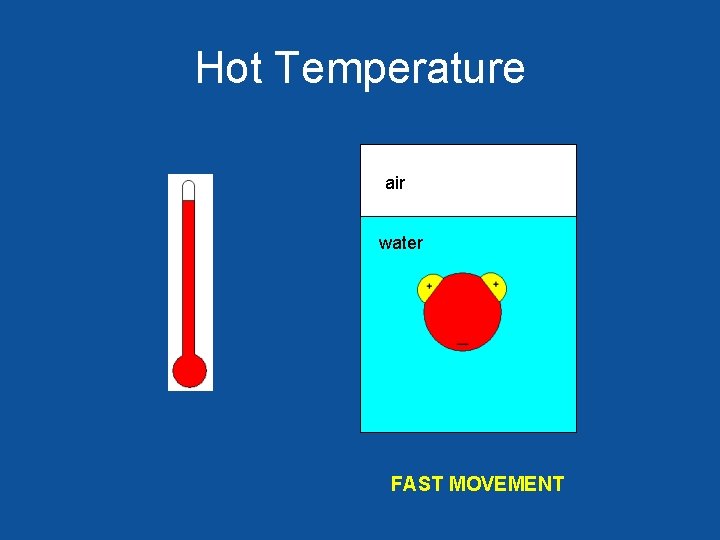
Hot Temperature air water FAST MOVEMENT

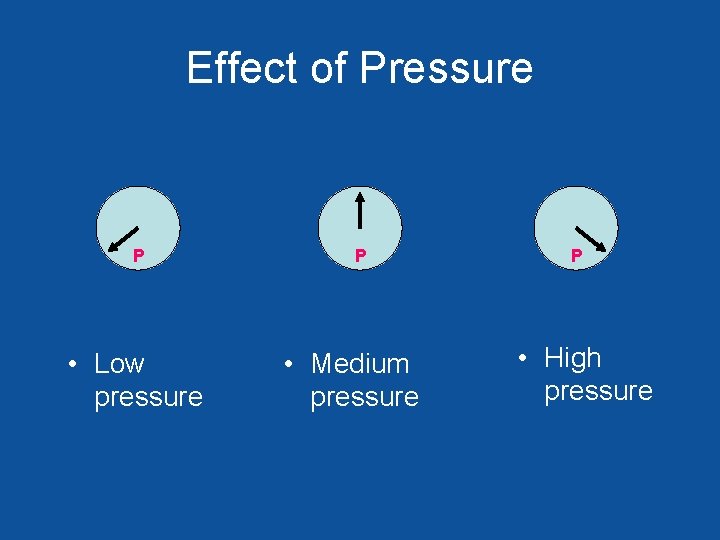
Effect of Pressure P • Low pressure P • Medium pressure P • High pressure

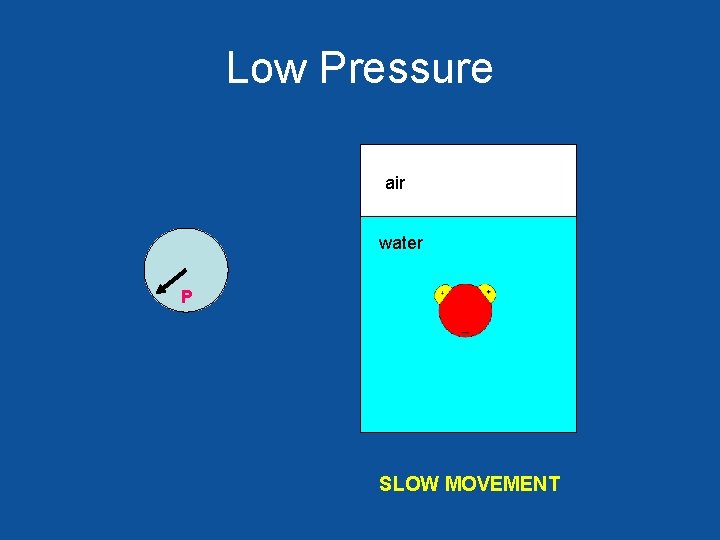
Low Pressure air water P SLOW MOVEMENT

Medium Temperature air water P MEDIUM MOVEMENT

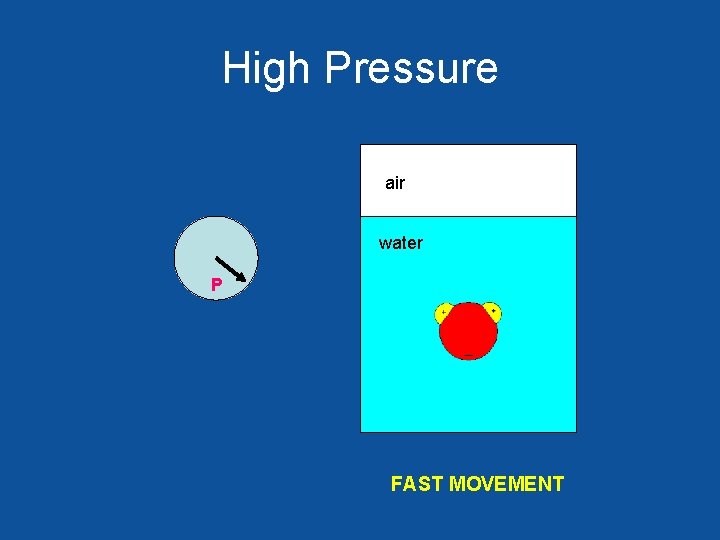
High Pressure air water P FAST MOVEMENT

To increase or decrease activity • Increase or decrease pressure • Temperature also counts, but minor effect over short distances

Chemical activity of molecules • Based on chemical environment – Determines what a molecule interacts with – Determines the effect of the interactions

Physical and chemical effects • Molecules have physical activity • Also chemical activity – Interact with other chemicals – Each molecule behaves in its own way • Water acts like water • Ca acts like Ca – What affects the chemical activity? – How much?

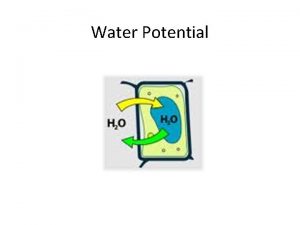
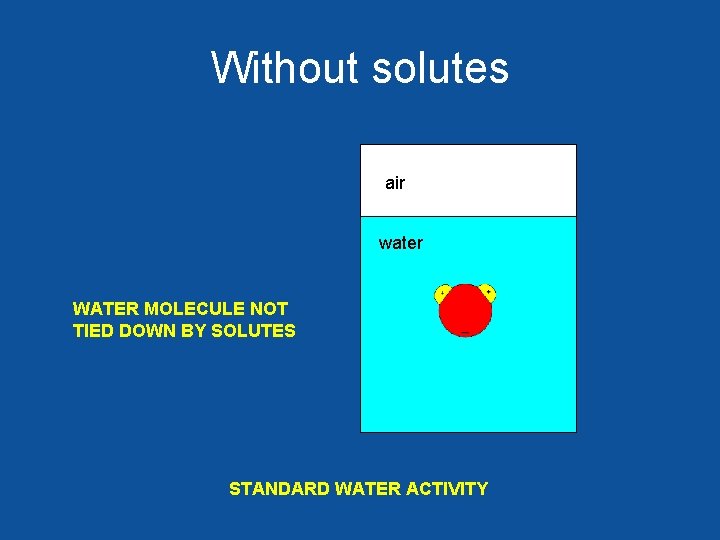
Without solutes air water WATER MOLECULE NOT TIED DOWN BY SOLUTES STANDARD WATER ACTIVITY

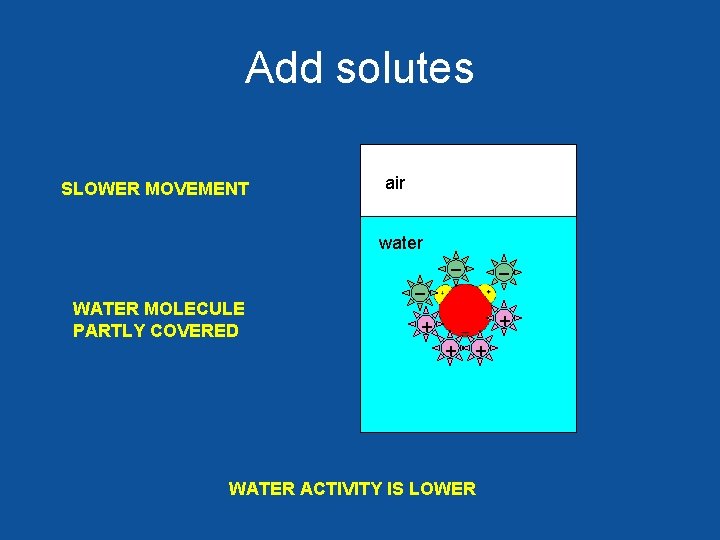
Add solutes SLOWER MOVEMENT air water _ _ _ WATER MOLECULE PARTLY COVERED + + WATER ACTIVITY IS LOWER

Change Water Activity • Increase – ↑ temperature – ↑ pressure • Decrease – ↓ temperature – ↓ pressure – add solutes • More solutes = more effect • But we are still focusing on water activity, not solute activity

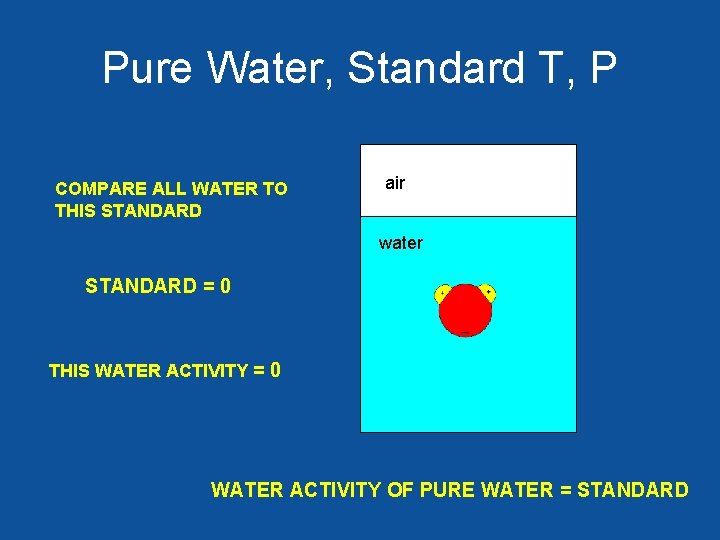
Pure Water, Standard T, P COMPARE ALL WATER TO THIS STANDARD air water STANDARD = 0 THIS WATER ACTIVITY = 0 WATER ACTIVITY OF PURE WATER = STANDARD

Water Potential • Water activity of your solution compared to water activity of standard solution • Standard solution = 0 • Your solution can be – Same activity (WP = 0) – Lower activity (WP < 0) – Higher activity (WP > 0) • Doesn’t occur in biology

Need to get some numbers • Water potential scale • Like temperature scale

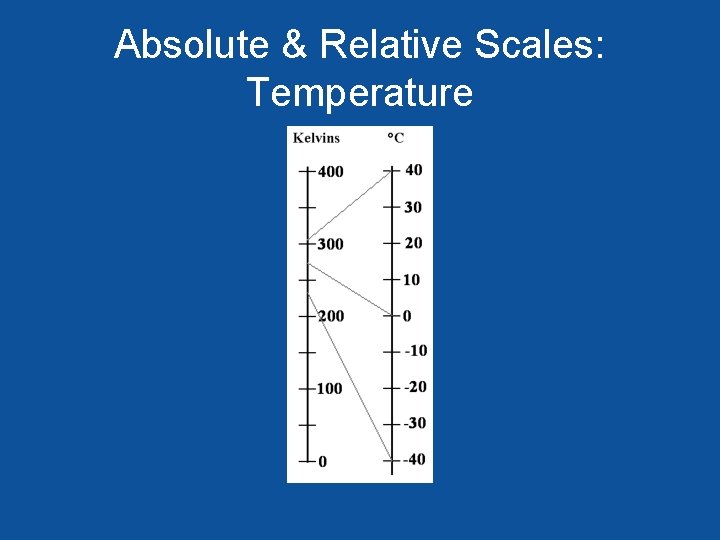
Absolute & Relative Scales: Temperature

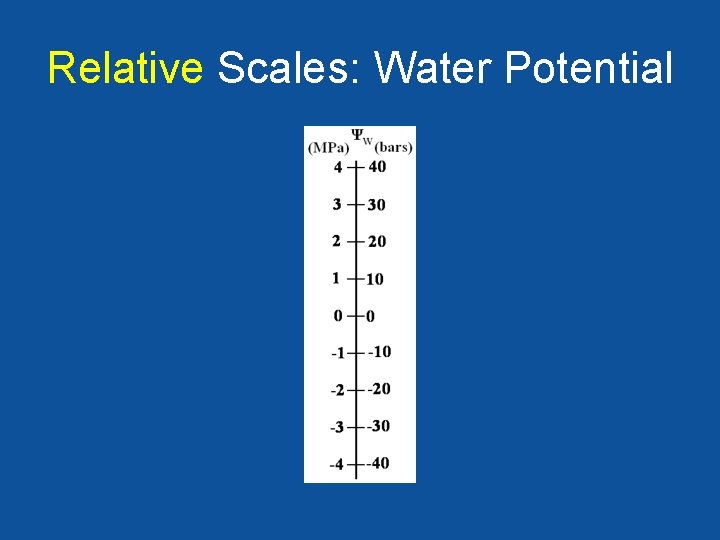
Relative Scales: Water Potential

Water potential scale • Water activity in cell (beaker, bucket, trunk) – Not the other chemicals • Distributed characteristic (all the same) – Like temperature • Compared to standard (0) • Can be positive, zero or negative • Expresses effect of P, T, solutes – On water, compared to standard

Summary • Physical activity of water molecules changes with – (Temperature, ) pressure • Chemical activity of water molecules changes with solutes • Water potential tells us the total activity – Compared to pure water, standard TP • WP can be +, 0, -

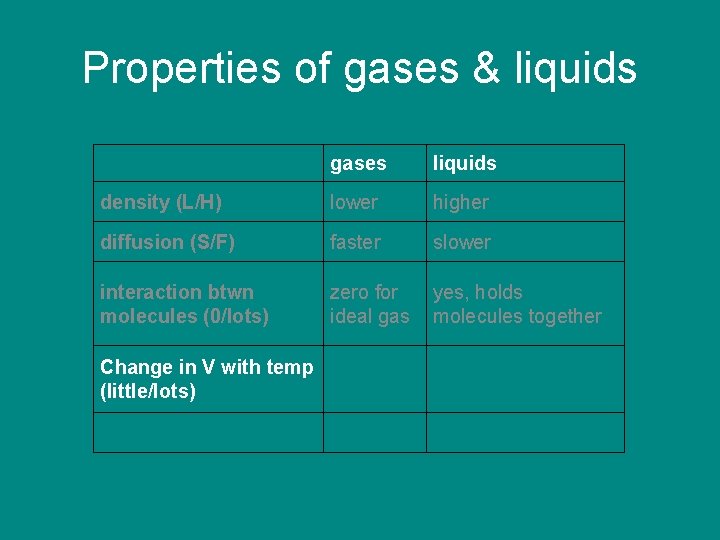
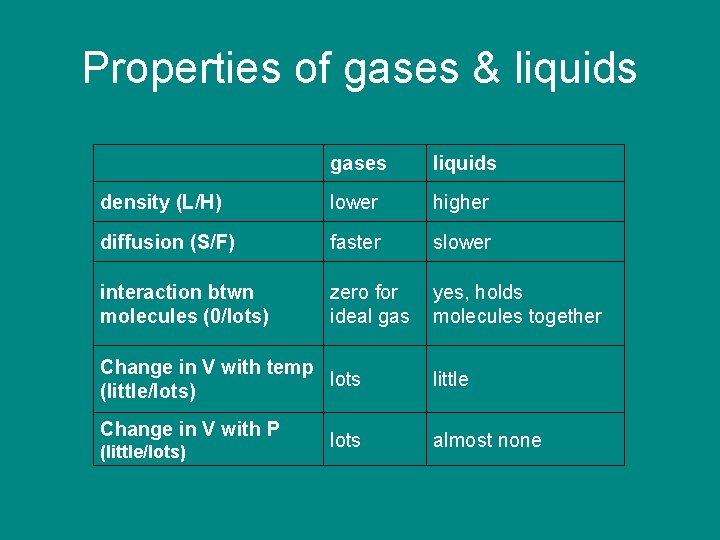
Properties of gases & liquids gases density (L/H) liquids

Properties of gases & liquids density (L/H) gases liquids lower higher

Properties of gases & liquids density (L/H) diffusion (S/F) gases liquids lower higher

Properties of gases & liquids gases liquids density (L/H) lower higher diffusion (S/F) faster slower

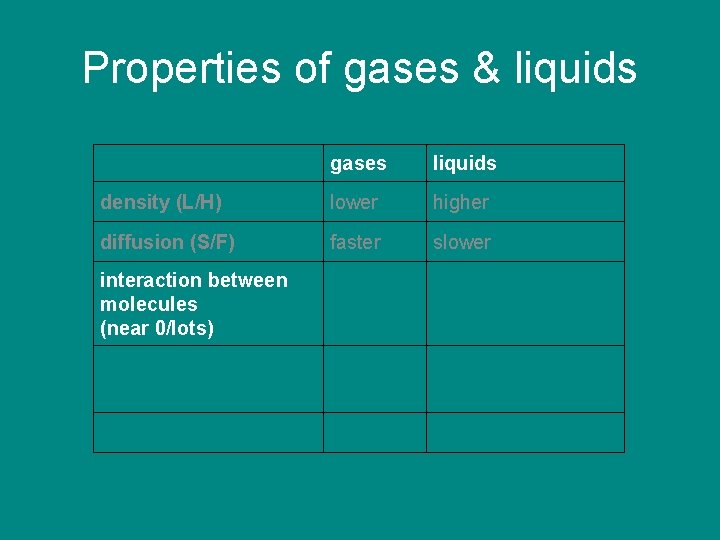
Properties of gases & liquids gases liquids density (L/H) lower higher diffusion (S/F) faster slower interaction between molecules (near 0/lots)

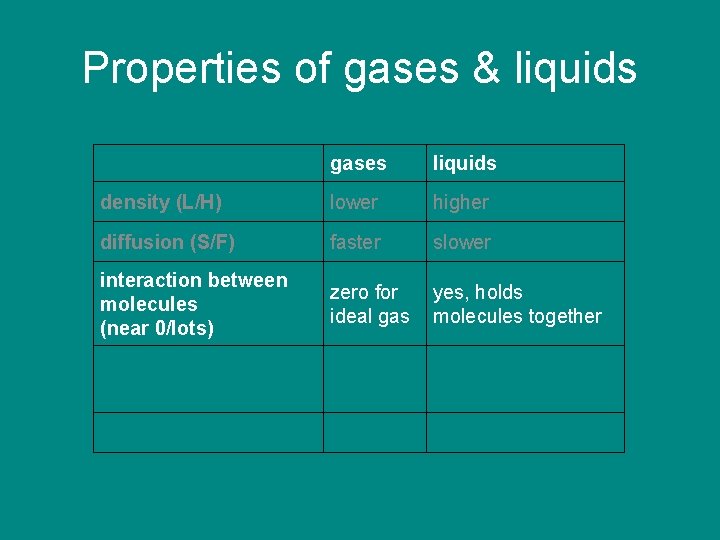
Properties of gases & liquids gases liquids density (L/H) lower higher diffusion (S/F) faster slower interaction between molecules (near 0/lots) zero for ideal gas yes, holds molecules together

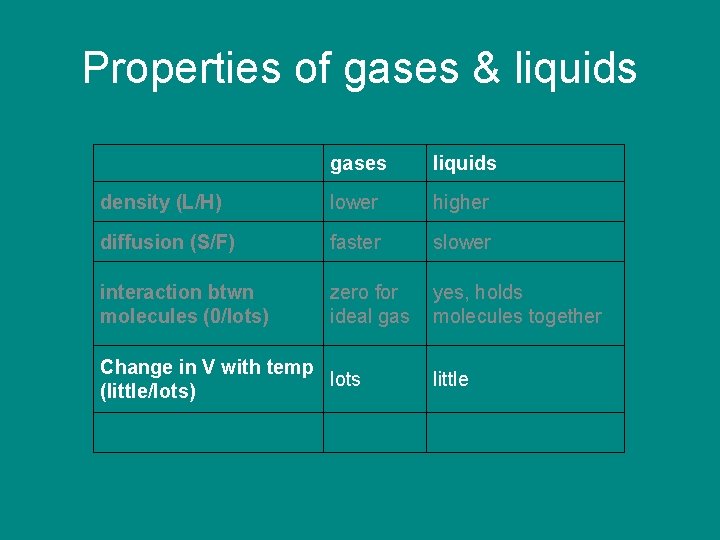
Properties of gases & liquids gases liquids density (L/H) lower higher diffusion (S/F) faster slower interaction btwn molecules (0/lots) zero for ideal gas yes, holds molecules together Change in V with temp (little/lots)

Properties of gases & liquids gases liquids density (L/H) lower higher diffusion (S/F) faster slower interaction btwn molecules (0/lots) zero for ideal gas yes, holds molecules together Change in V with temp lots (little/lots) little

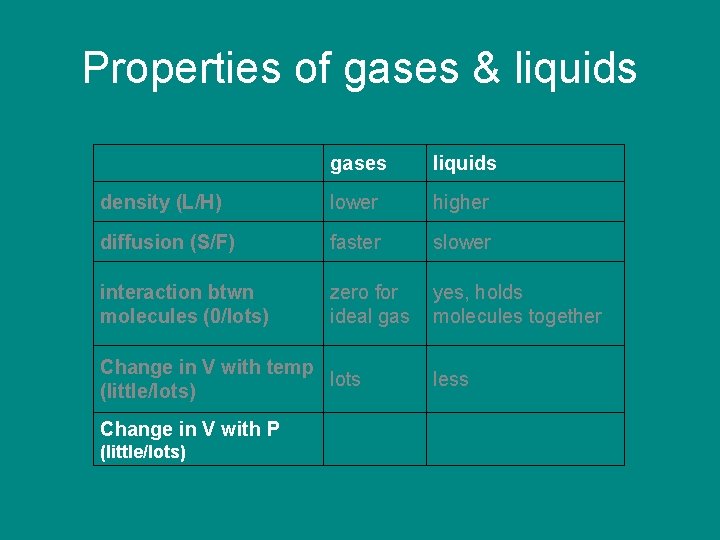
Properties of gases & liquids gases liquids density (L/H) lower higher diffusion (S/F) faster slower interaction btwn molecules (0/lots) zero for ideal gas yes, holds molecules together Change in V with temp lots (little/lots) Change in V with P (little/lots) less

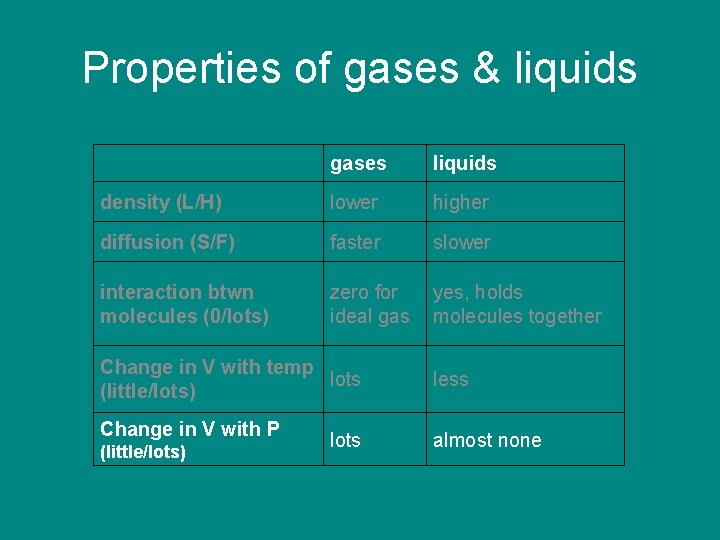
Properties of gases & liquids gases liquids density (L/H) lower higher diffusion (S/F) faster slower interaction btwn molecules (0/lots) zero for ideal gas yes, holds molecules together Change in V with temp lots (little/lots) Change in V with P (little/lots) lots less almost none

Properties of gases & liquids gases liquids density (L/H) lower higher diffusion (S/F) faster slower interaction btwn molecules (0/lots) zero for ideal gas yes, holds molecules together Change in V with temp lots (little/lots) Change in V with P (little/lots) lots little almost none

Water: gas, liquid & solid • • Life mostly water: properties critical Boil or evaporate: big change in volume Small volume change when we make ice Polar molecules – Not stuck together in gas – Stuck together loosely in liquid – Stuck together tightly in solid

Acting out a gas • Student volunteers • Gas molecules don’t hold onto each other • Can compress if contained (can push) – big volume change • Can't pull (no connection between molecules) • Gases: can push but not pull • Gases: under positive pressure only

Acting out a liquid (water) • Same volunteers • Can push or pull: molecules bound to each other – Especially true for water – More like solid than like gas • Positive or negative P (“pressure” or “tension”) – Compression: P is + – Tension: P is - (like solid) • Must contain liquid to push (like gas)

Gas in liquid—acting out in class • Same volunteers • Make the middle molecule a gas – Can push – Can't pull • Gas bubble is called embolism – may occur naturally • Embolisms stop transport based on pulling

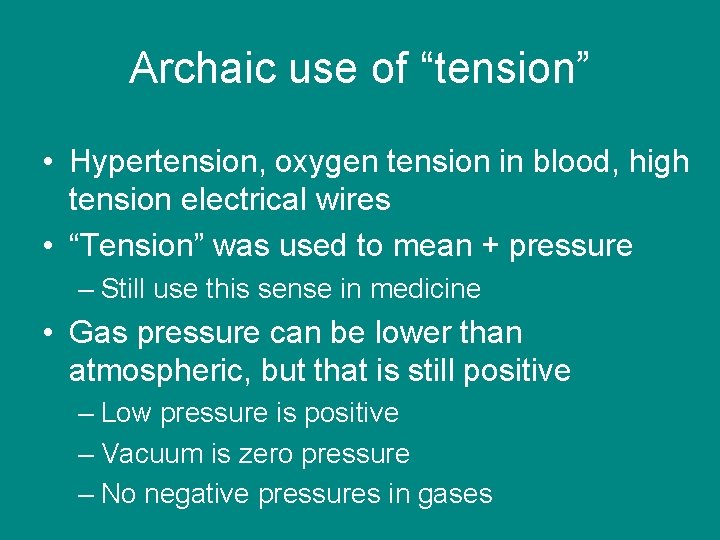
Archaic use of “tension” • Hypertension, oxygen tension in blood, high tension electrical wires • “Tension” was used to mean + pressure – Still use this sense in medicine • Gas pressure can be lower than atmospheric, but that is still positive – Low pressure is positive – Vacuum is zero pressure – No negative pressures in gases

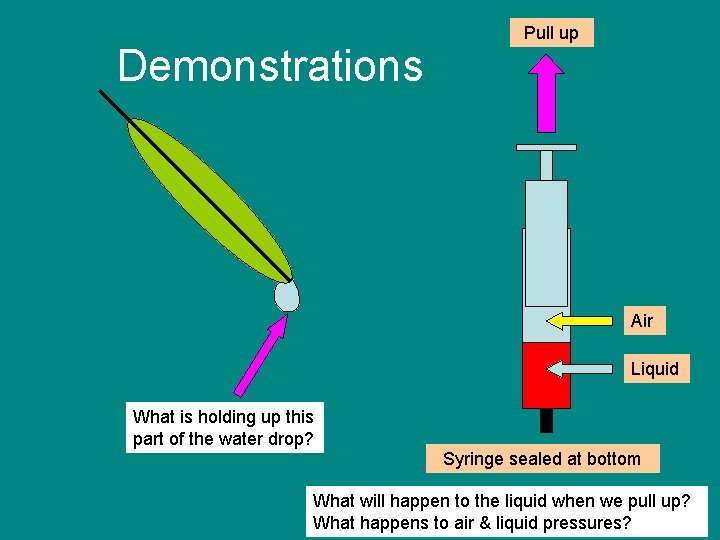
Demonstrations Pull up Air Liquid What is holding up this part of the water drop? Syringe sealed at bottom What will happen to the liquid when we pull up? What happens to air & liquid pressures?
 Osmotic potential vs water potential
Osmotic potential vs water potential How to find pressure potential
How to find pressure potential ψs
ψs How to find pressure potential
How to find pressure potential Water and water and water water
Water and water and water water Physiology
Physiology Plant physiology
Plant physiology Salisbury and ross plant physiology
Salisbury and ross plant physiology Graded potential vs action potential
Graded potential vs action potential Decremental graded potential
Decremental graded potential Graded potential vs action potential
Graded potential vs action potential Action potential definition
Action potential definition Difference between action and graded potential
Difference between action and graded potential Sources of bioelectric potentials
Sources of bioelectric potentials Transmission across a synapse
Transmission across a synapse Suggmadex
Suggmadex Action potential resting potential
Action potential resting potential Action potential resting potential
Action potential resting potential Sales potential vs market potential
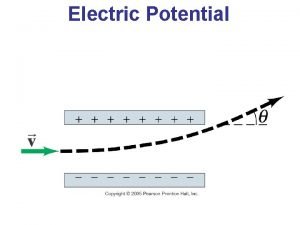
Sales potential vs market potential Equipotential lines
Equipotential lines Potential unit
Potential unit Electric potential inside non conducting sphere
Electric potential inside non conducting sphere Electric potential from electric field
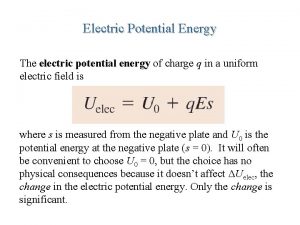
Electric potential from electric field Potential energy of an electric field
Potential energy of an electric field V = pe/q
V = pe/q Electrical potential
Electrical potential Absolute refractory period and relative refractory period
Absolute refractory period and relative refractory period A&p flix activity: resting membrane potential
A&p flix activity: resting membrane potential A&p flix activity: resting membrane potential
A&p flix activity: resting membrane potential Sem vi
Sem vi Plant introduction in plant breeding
Plant introduction in plant breeding Plant introduction in plant breeding
Plant introduction in plant breeding Tronsmo plant pathology and plant diseases download
Tronsmo plant pathology and plant diseases download Tronsmo plant pathology and plant diseases download
Tronsmo plant pathology and plant diseases download Tronsmo plant pathology and plant diseases download
Tronsmo plant pathology and plant diseases download Uni due water science klausuren
Uni due water science klausuren Activity 2 limiting reactants activity
Activity 2 limiting reactants activity Activity corrections
Activity corrections How to draw aoa diagram
How to draw aoa diagram Activity 1 introductory activity
Activity 1 introductory activity Activity 2 finding the sequence
Activity 2 finding the sequence Activity 2 plus and minus
Activity 2 plus and minus 1index
1index