Water Potential p s Water potential pressure potential






- Slides: 12

Water Potential

Ψ = Ψp + Ψs Water potential = pressure potential + solute potential *Bozeman science video

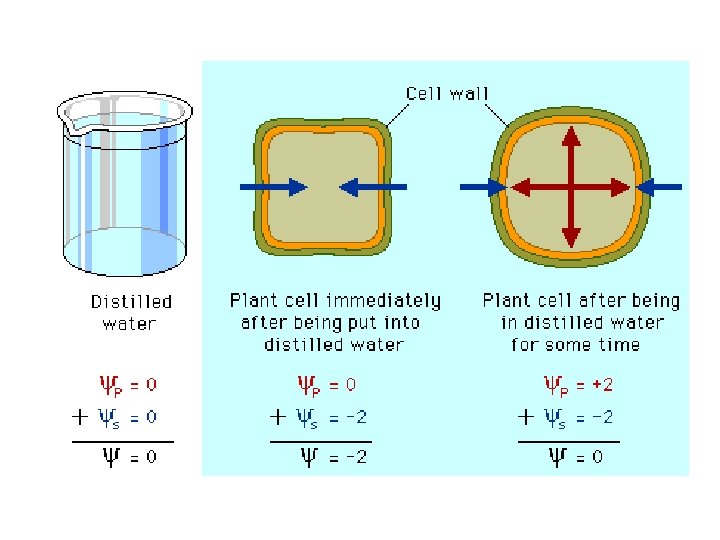
Water Potential • Water Potential - In osmosis, the tendency for a system (a cell or solution) to take up water from pure water, through a differentially permeable membrane. • Water will ALWAYS move from a region of HIGH water potential to an area of LOW water potential.

Ψp (PRESSURE POTENTIAL) – An increase in pressure INCREASES water potential – Normal atmospheric pressure: Ψp = 0

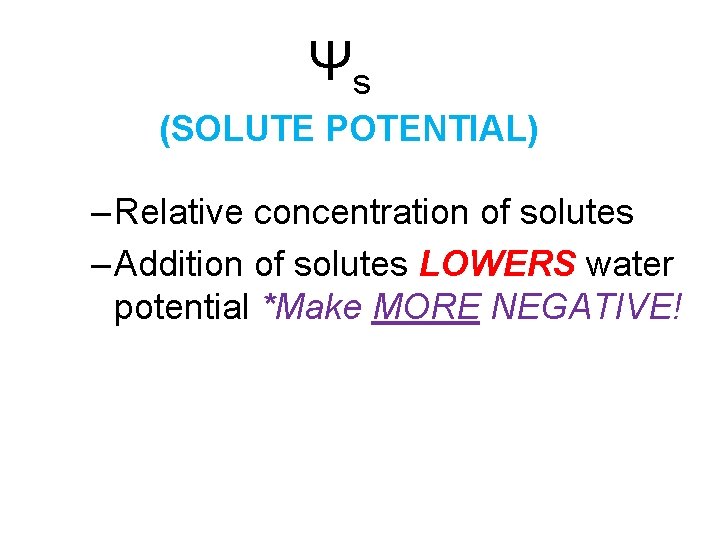
Ψs (SOLUTE POTENTIAL) – Relative concentration of solutes – Addition of solutes LOWERS water potential *Make MORE NEGATIVE!

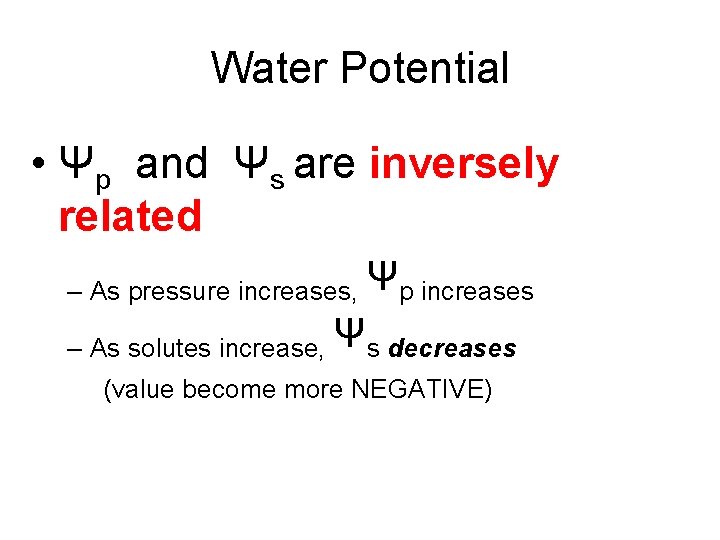
Water Potential • Ψp and Ψs are inversely related – As pressure increases, Ψp increases – As solutes increase, Ψs decreases (value become more NEGATIVE)


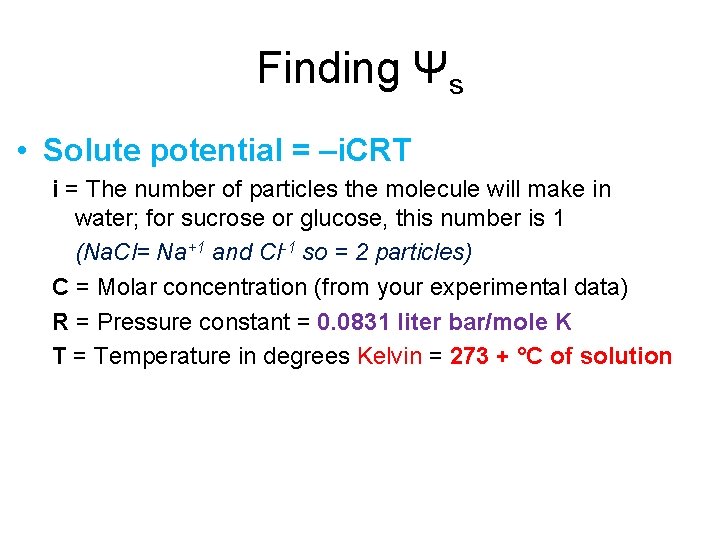
Finding Ψs • Solute potential = –i. CRT i = The number of particles the molecule will make in water; for sucrose or glucose, this number is 1 (Na. Cl= Na+1 and Cl-1 so = 2 particles) C = Molar concentration (from your experimental data) R = Pressure constant = 0. 0831 liter bar/mole K T = Temperature in degrees Kelvin = 273 + °C of solution

Finding Ψs • The molar concentration of a sugar solution in an open beaker has been determined to be 0. 3 M. Calculate the solute potential at 27 °C degrees. Round your answer to the nearest hundredth.

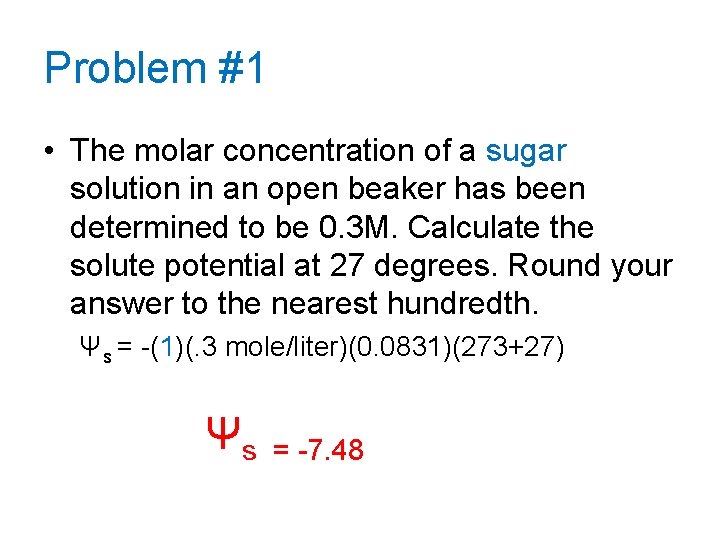
Problem #1 • The molar concentration of a sugar solution in an open beaker has been determined to be 0. 3 M. Calculate the solute potential at 27 degrees. Round your answer to the nearest hundredth. Ψs = -(1)(. 3 mole/liter)(0. 0831)(273+27) Ψs = -7. 48

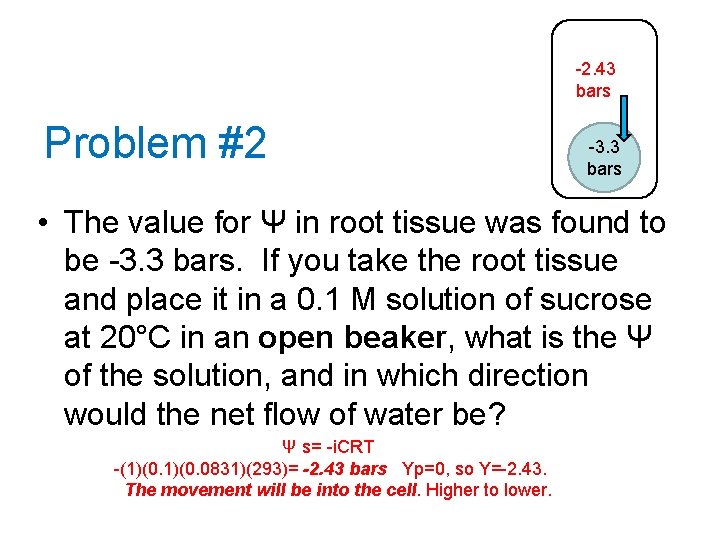
-2. 43 bars Problem #2 -3. 3 bars • The value for Ψ in root tissue was found to be -3. 3 bars. If you take the root tissue and place it in a 0. 1 M solution of sucrose at 20°C in an open beaker, what is the Ψ of the solution, and in which direction would the net flow of water be? Ψ s= -i. CRT -(1)(0. 0831)(293)= -2. 43 bars Yp=0, so Y=-2. 43. The movement will be into the cell. Higher to lower.

Problem #3 -4. 86 bars -3. 3 bars • Na. Cl dissociates into 2 particles in water: Na+ and Cl-. If the solution in question 4 contained 0. 1 M Na. Cl instead of 0. 1 M sucrose, what is the Ψ of the solution, and in which direction would the net flow of water be? -(2)(0. 1)(0. 0831)(293) + 0= Ψ= -4. 86 Into the environment