A Pilot Study of PLX 3397 a Selective




- Slides: 23

A Pilot Study of PLX 3397, a Selective Colony-Stimulating Factor 1 Receptor (CSF 1 R) Kinase Inhibitor, in Pigmented Villonodular Synovitis (PVNS) Presented By William Tap at 2014 ASCO Annual Meeting

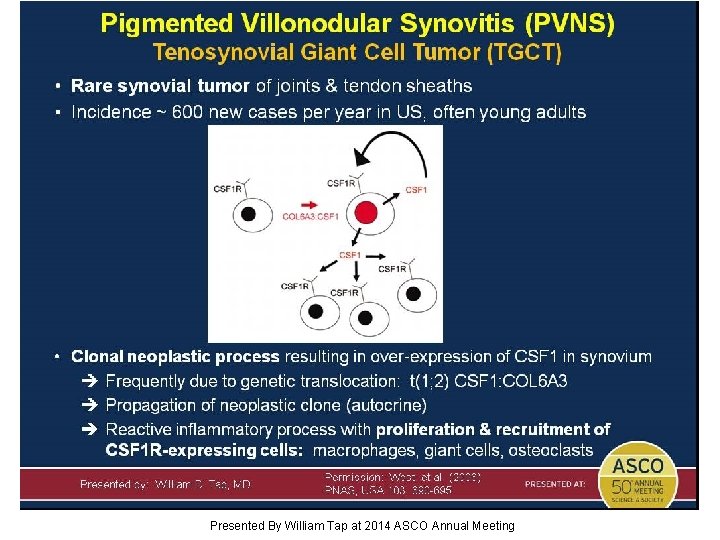
Pigmented Villonodular Synovitis (PVNS) Tenosynovial Giant Cell Tumor (TGCT) Presented By William Tap at 2014 ASCO Annual Meeting

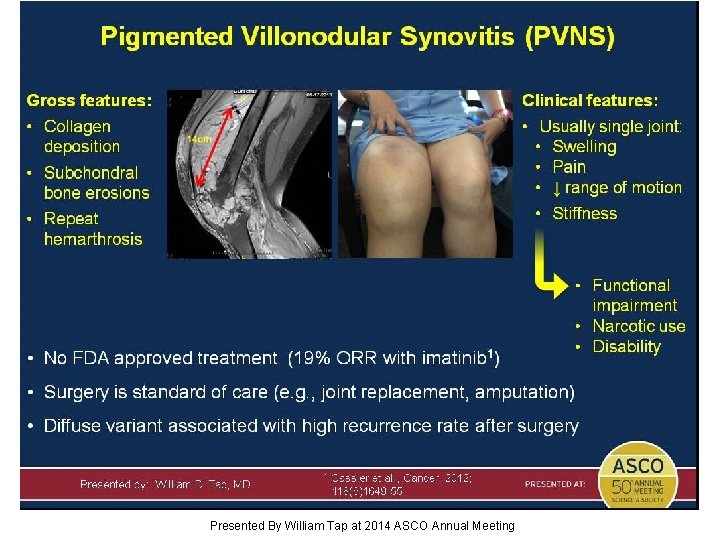
Pigmented Villonodular Synovitis (PVNS) Presented By William Tap at 2014 ASCO Annual Meeting

Slide 4 Presented By William Tap at 2014 ASCO Annual Meeting

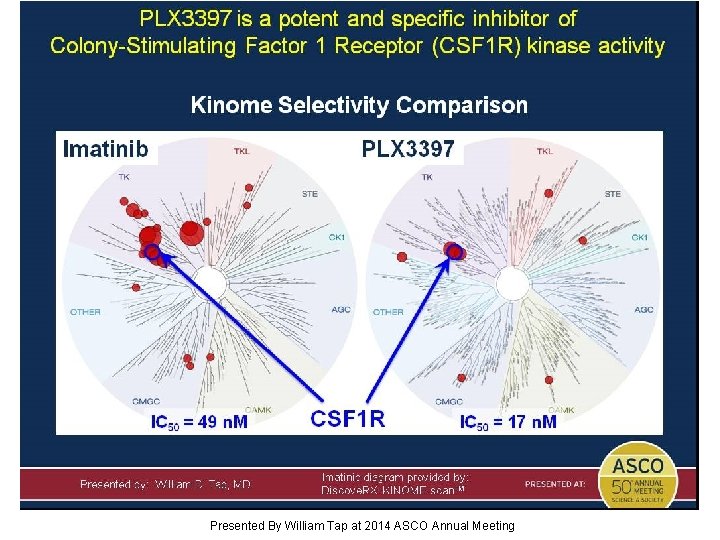
Can we help patients (PVNS) with a highly targeted therapy (PLX 3397) that blocks the CSF 1 R pathway in this clonal neoplastic process frequently initiated by a single genetic event? Presented By William Tap at 2014 ASCO Annual Meeting

Study Goals Presented By William Tap at 2014 ASCO Annual Meeting

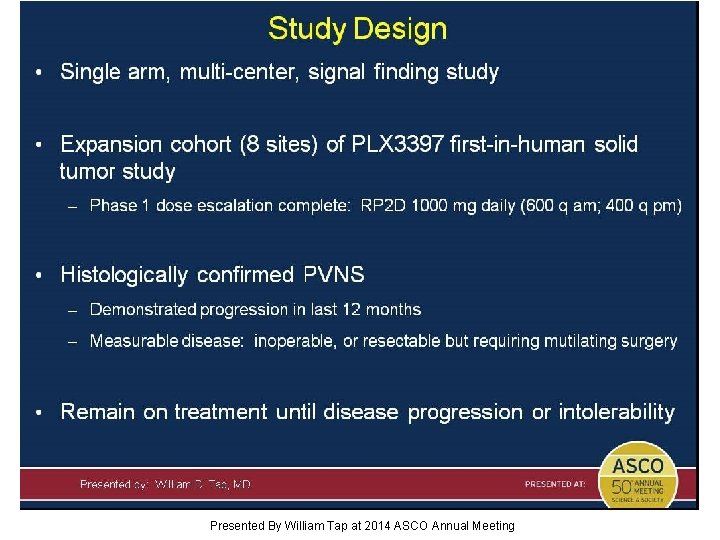
Study Design Presented By William Tap at 2014 ASCO Annual Meeting

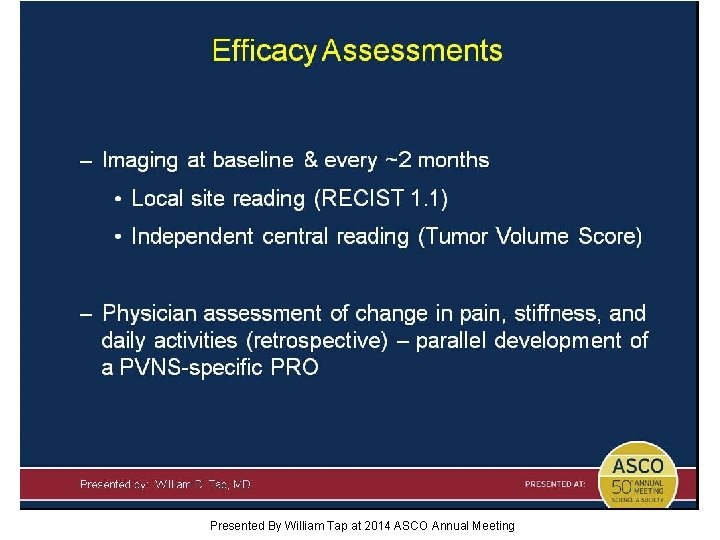
Efficacy Assessments Presented By William Tap at 2014 ASCO Annual Meeting

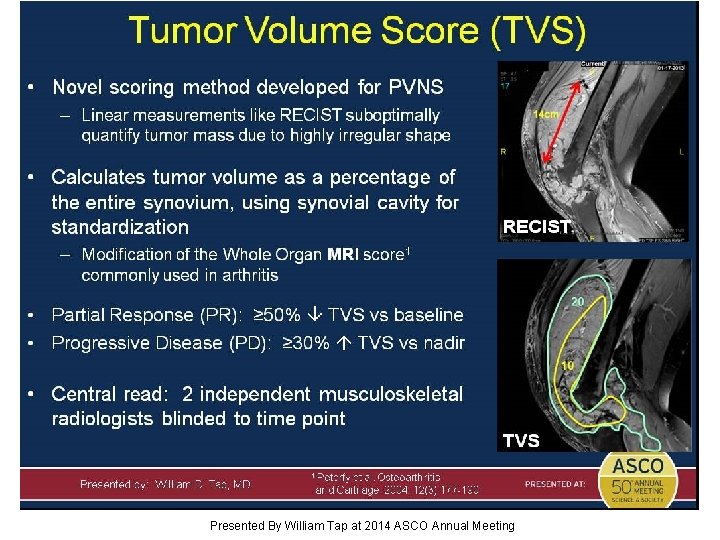
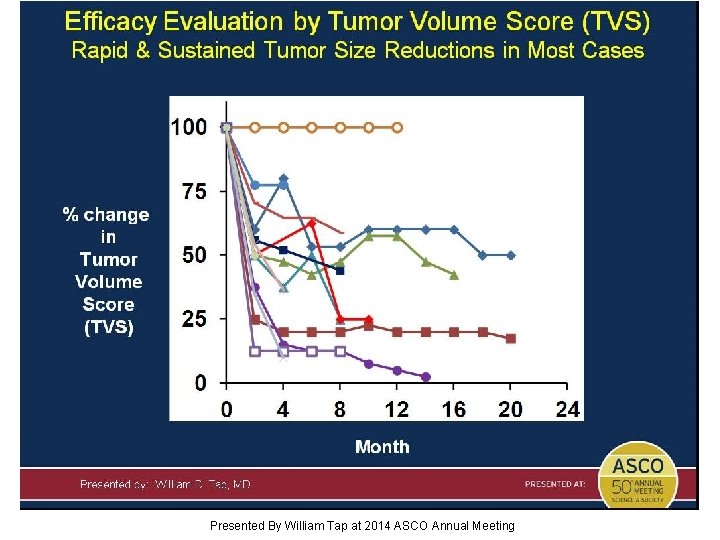
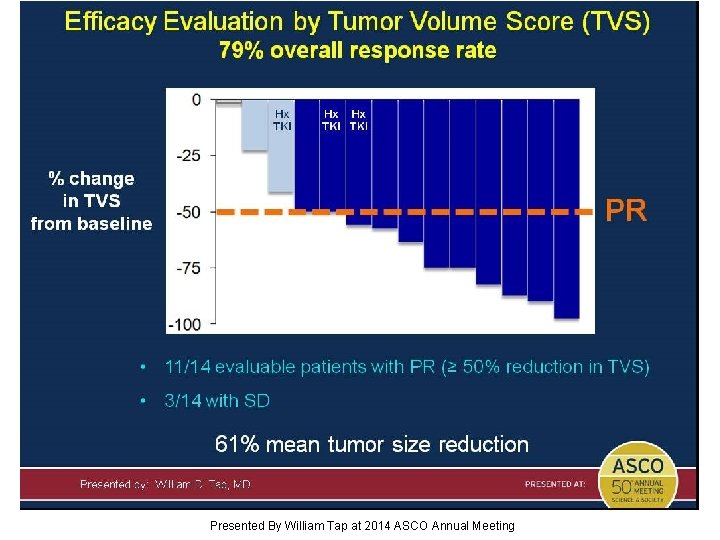
Tumor Volume Score (TVS) Presented By William Tap at 2014 ASCO Annual Meeting

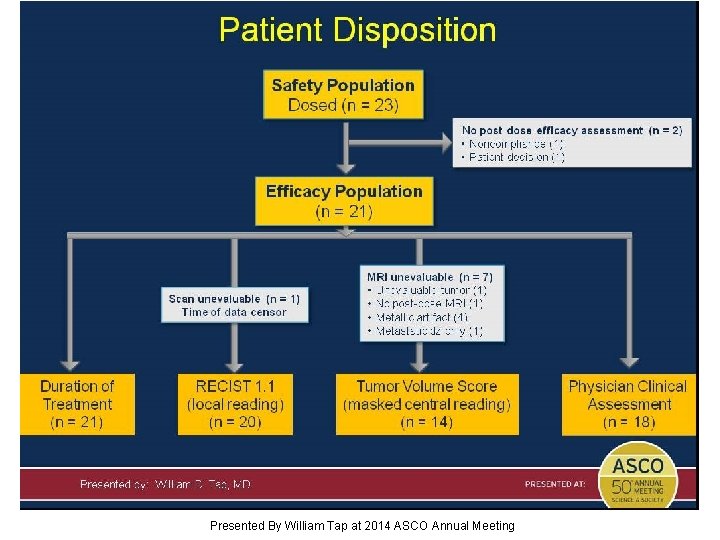
Patient Disposition Presented By William Tap at 2014 ASCO Annual Meeting

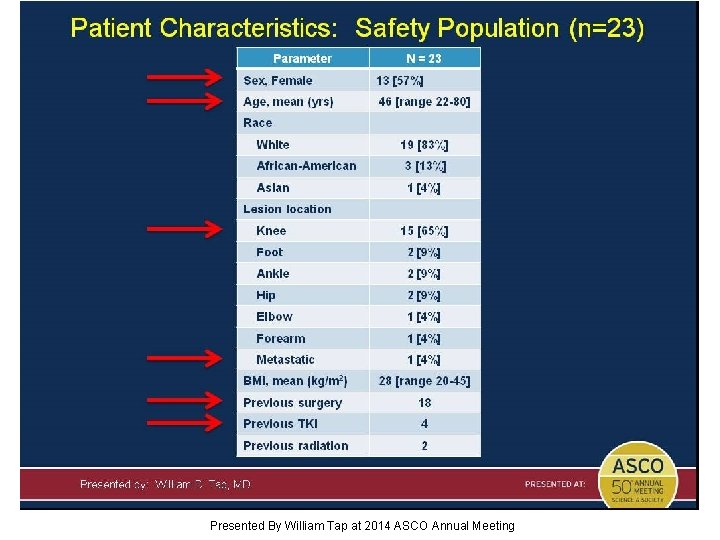
Patient Characteristics: Safety Population (n=23) Presented By William Tap at 2014 ASCO Annual Meeting

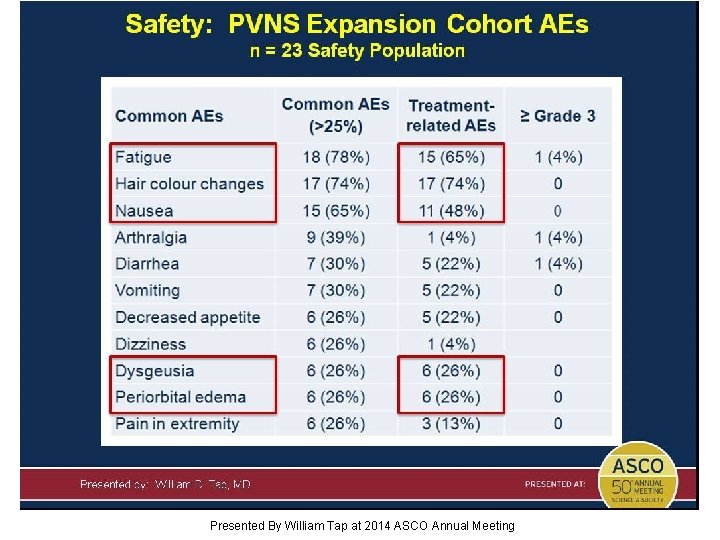
Safety: PVNS Expansion Cohort AEs n = 23 Safety Population Presented By William Tap at 2014 ASCO Annual Meeting

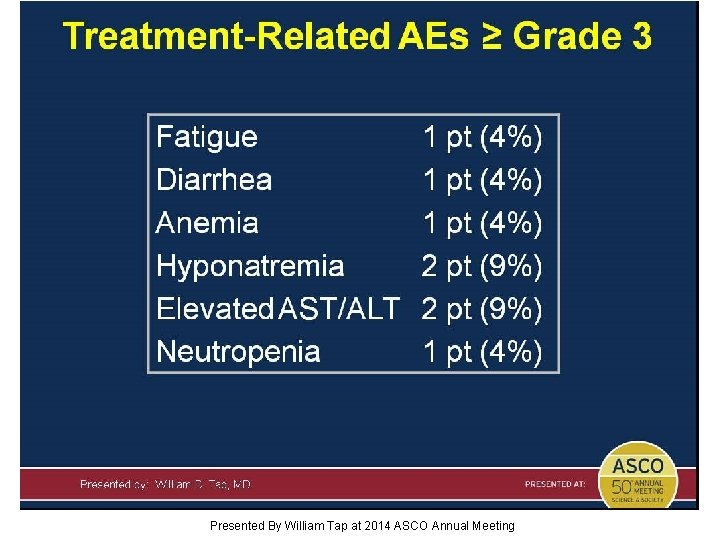
Treatment-Related AEs ≥ Grade 3 Presented By William Tap at 2014 ASCO Annual Meeting

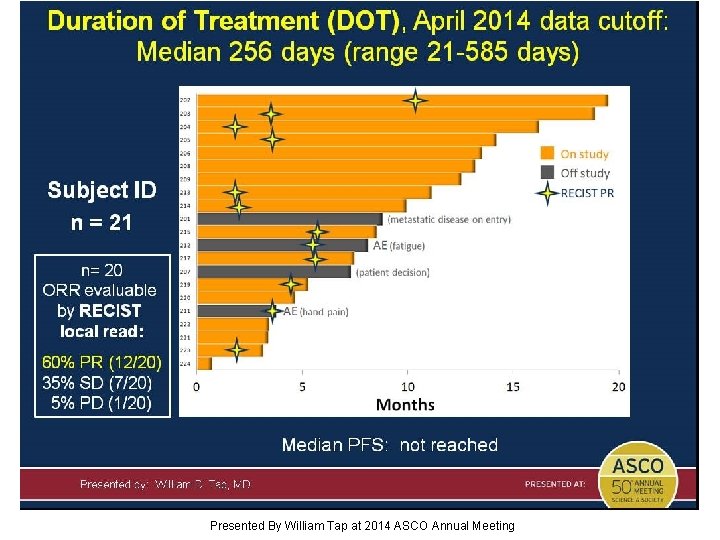
Duration of Treatment (DOT), April 2014 data cutoff: Median 256 days (range 21 -585 days) Presented By William Tap at 2014 ASCO Annual Meeting

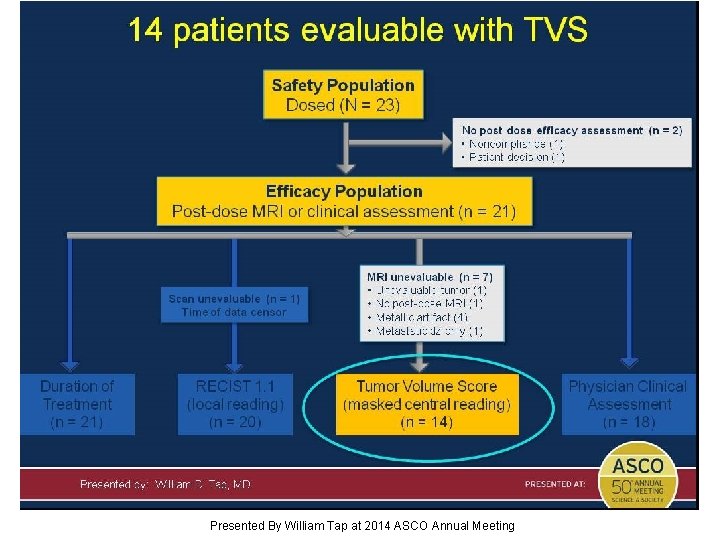
14 patients evaluable with TVS Presented By William Tap at 2014 ASCO Annual Meeting

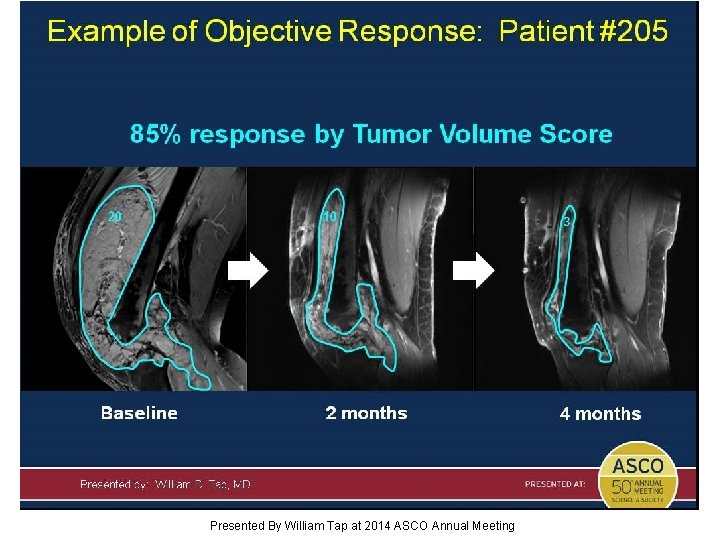
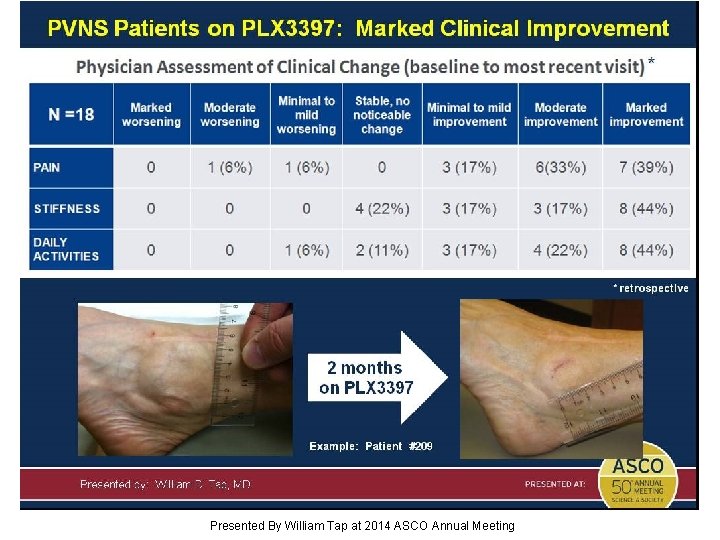
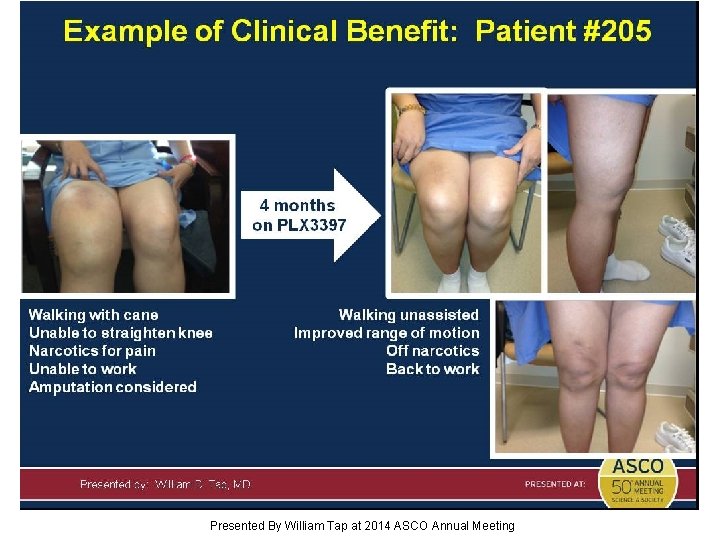
Slide 16 Presented By William Tap at 2014 ASCO Annual Meeting

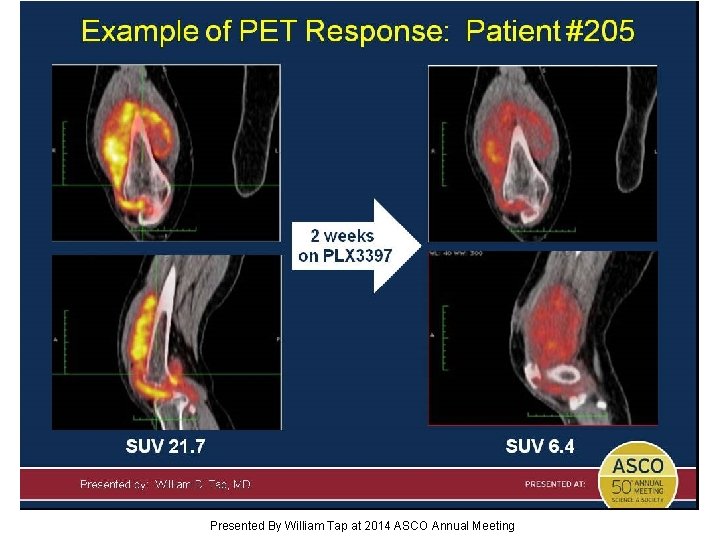
Slide 17 Presented By William Tap at 2014 ASCO Annual Meeting

Slide 18 Presented By William Tap at 2014 ASCO Annual Meeting

Slide 19 Presented By William Tap at 2014 ASCO Annual Meeting

Slide 20 Presented By William Tap at 2014 ASCO Annual Meeting

Slide 21 Presented By William Tap at 2014 ASCO Annual Meeting

Conclusions Presented By William Tap at 2014 ASCO Annual Meeting

Thank You Presented By William Tap at 2014 ASCO Annual Meeting