A FirstPrinciples Study of Thiol Ligated Cd Se









- Slides: 34

A First-Principles Study of Thiol Ligated Cd. Se Nanoclusters Shanshan Wu 1 Aug 1 st, 2012 Advisor: James Glimm 1, 2 Collaborators: Michael Mc. Guigan 2, Stan Wong 1, 2, Amanda Tiano 1 1. Stony Brook University 2. Brookhaven National Laboratory

Outline Introduction � Computational Model � Results and Discussions � Conclusions and Prospects � 2

Outline Introduction � Computational Model � Results and Discussions � Conclusions and Prospects � 3

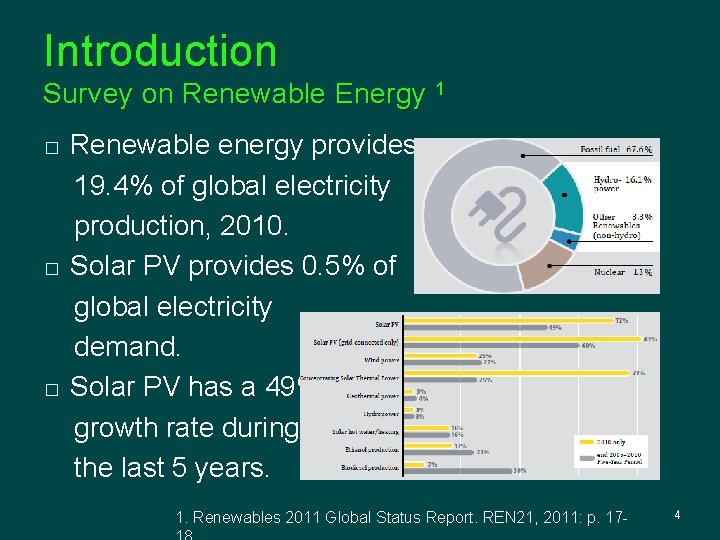
Introduction Survey on Renewable Energy 1 � � � Renewable energy provides 19. 4% of global electricity production, 2010. Solar PV provides 0. 5% of global electricity demand. Solar PV has a 49% growth rate during the last 5 years. 1. Renewables 2011 Global Status Report. REN 21, 2011: p. 17 - 4

Introduction Profile of Quantum Dot(QD) Sensitized Solar Cell � Advantage 1 • Tailor the absorption spectrum by size control. • Low-cost production method � 12% experimental efficiency 2 � Research Interests • Size and Shape Control of QDs • Surface Passivation • Attachment and Electron Transmission to the Ti. O 2 1. Rühle, S. , et al. , Chem. Phys. Chem, 2010. 11(11): p. 2290 -2304. 2. Robel, I. , et al. , J. Am. Chem. Soc, 2006. 128(7): p. 2385 -2393. 5

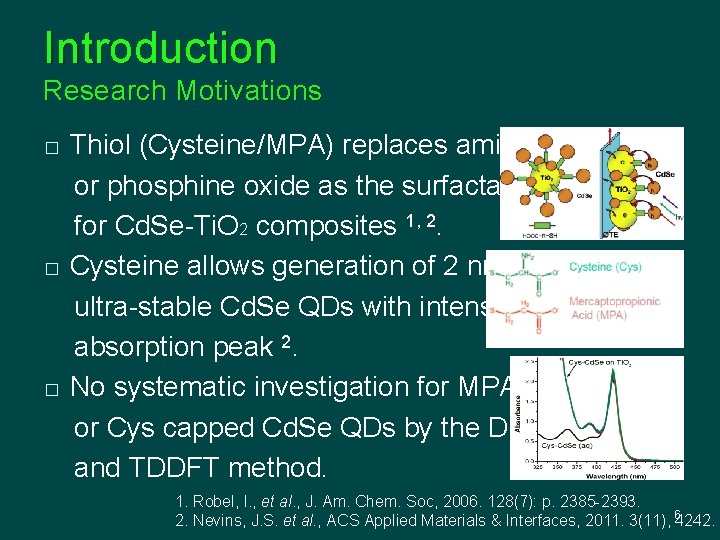
Introduction Research Motivations � � � Thiol (Cysteine/MPA) replaces amine or phosphine oxide as the surfactant for Cd. Se-Ti. O 2 composites 1, 2. Cysteine allows generation of 2 nm ultra-stable Cd. Se QDs with intensive absorption peak 2. No systematic investigation for MPA or Cys capped Cd. Se QDs by the DFT and TDDFT method. 1. Robel, I. , et al. , J. Am. Chem. Soc, 2006. 128(7): p. 2385 -2393. 2. Nevins, J. S. et al. , ACS Applied Materials & Interfaces, 2011. 3(11), 64242.

Outline Introduction � Computational Model � Results and Discussions � Conclusions and Prospects � 7

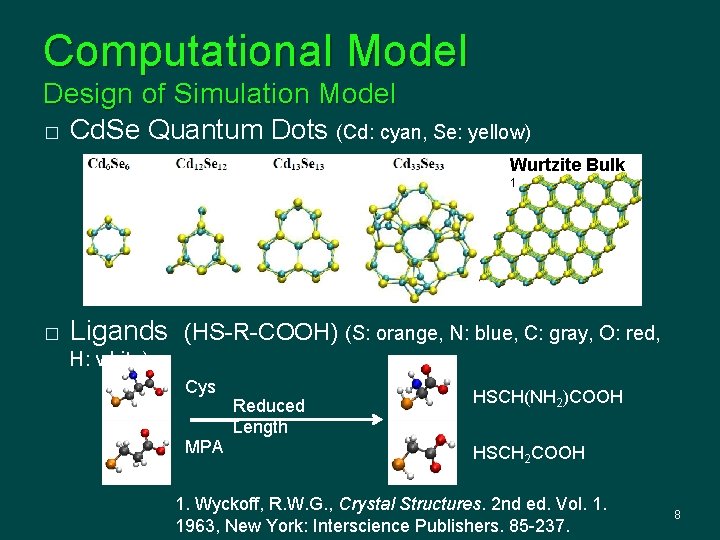
Computational Model Design of Simulation Model � Cd. Se Quantum Dots (Cd: cyan, Se: yellow) Wurtzite Bulk 1 � Ligands (HS-R-COOH) (S: orange, N: blue, C: gray, O: red, H: white) Cys MPA Reduced Length HSCH(NH 2)COOH HSCH 2 COOH 1. Wyckoff, R. W. G. , Crystal Structures. 2 nd ed. Vol. 1. 1963, New York: Interscience Publishers. 85 -237. 8

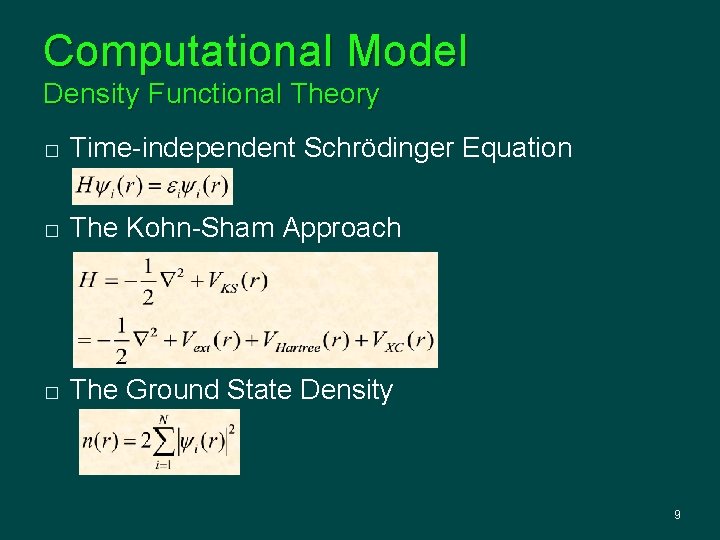
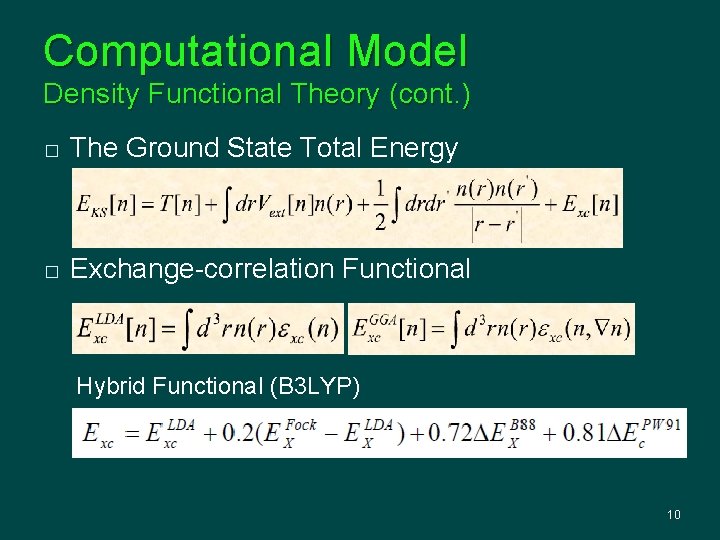
Computational Model Density Functional Theory � Time-independent Schrödinger Equation � The Kohn-Sham Approach � The Ground State Density 9

Computational Model Density Functional Theory (cont. ) � The Ground State Total Energy � Exchange-correlation Functional Hybrid Functional (B 3 LYP) 10

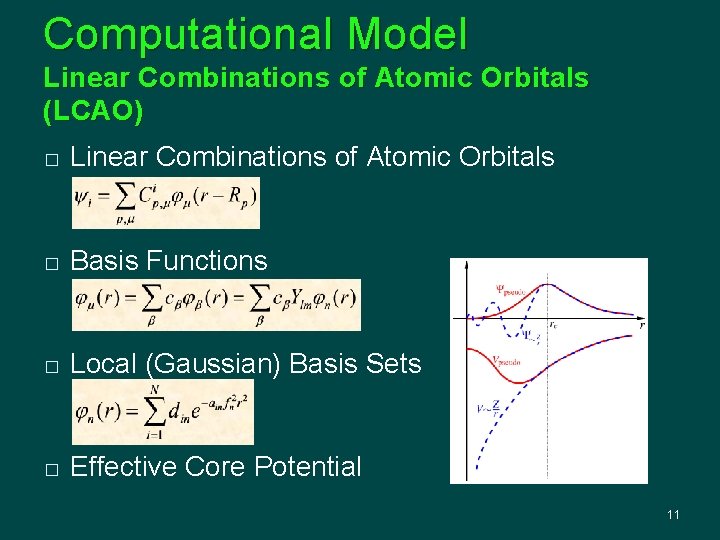
Computational Model Linear Combinations of Atomic Orbitals (LCAO) � Linear Combinations of Atomic Orbitals � Basis Functions � Local (Gaussian) Basis Sets � Effective Core Potential 11

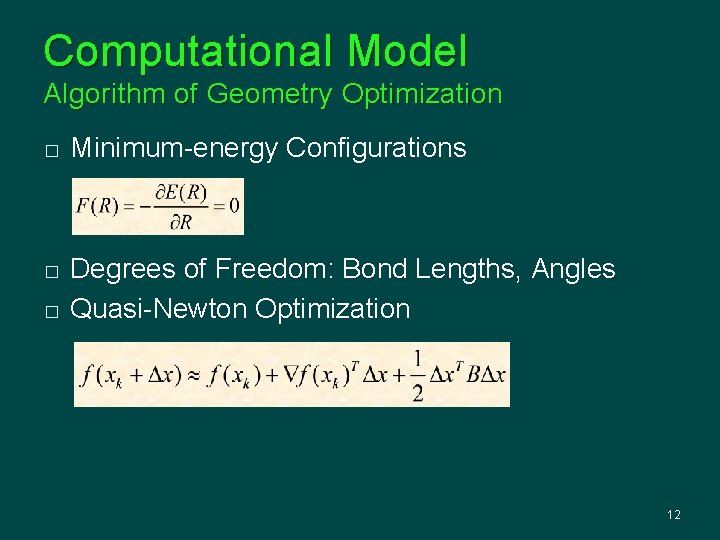
Computational Model Algorithm of Geometry Optimization � � � Minimum-energy Configurations Degrees of Freedom: Bond Lengths, Angles Quasi-Newton Optimization 12

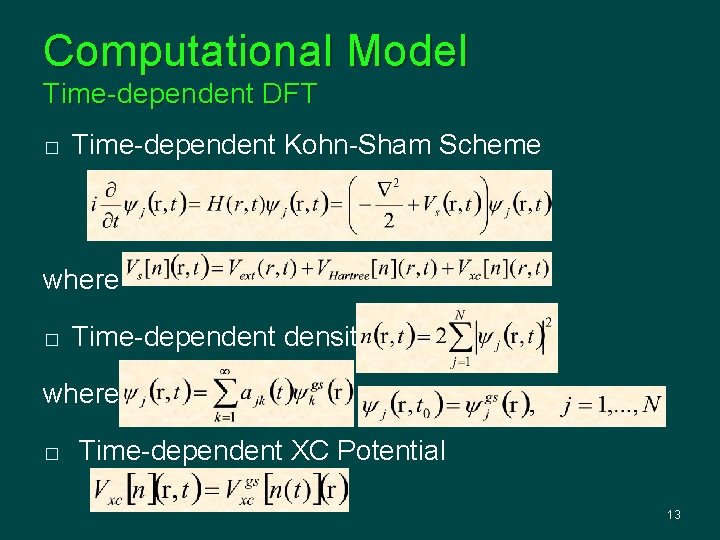
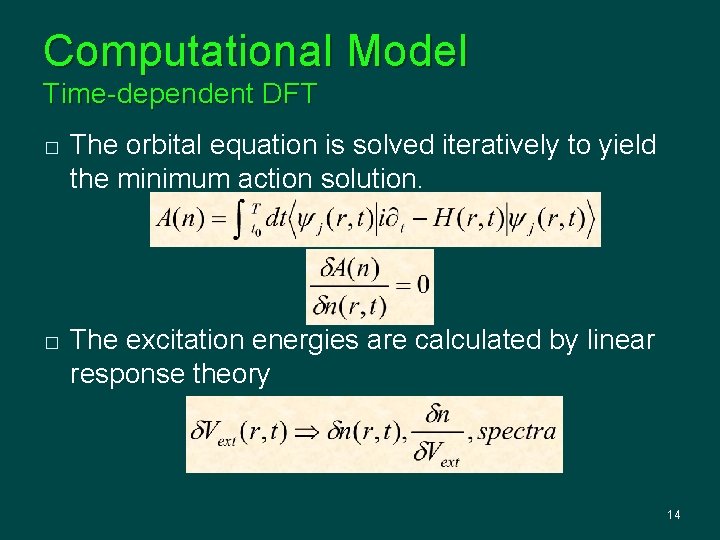
Computational Model Time-dependent DFT � Time-dependent Kohn-Sham Scheme where � Time-dependent density: where � Time-dependent XC Potential 13

Computational Model Time-dependent DFT � � The orbital equation is solved iteratively to yield the minimum action solution. The excitation energies are calculated by linear response theory 14

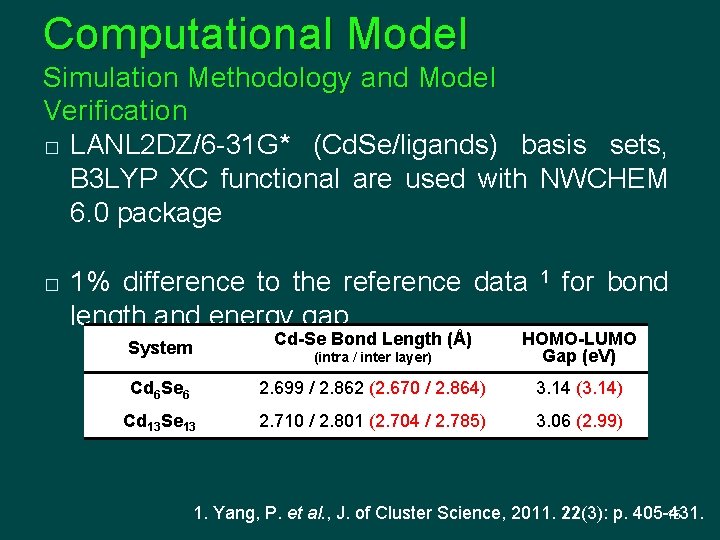
Computational Model Simulation Methodology and Model Verification � LANL 2 DZ/6 -31 G* (Cd. Se/ligands) basis sets, B 3 LYP XC functional are used with NWCHEM 6. 0 package � 1% difference to the reference data length and energy gap 1 for bond System Cd-Se Bond Length (Å) (intra / inter layer) HOMO-LUMO Gap (e. V) Cd 6 Se 6 2. 699 / 2. 862 (2. 670 / 2. 864) 3. 14 (3. 14) Cd 13 Se 13 2. 710 / 2. 801 (2. 704 / 2. 785) 3. 06 (2. 99) 1. Yang, P. et al. , J. of Cluster Science, 2011. 22(3): p. 405 -431. 15

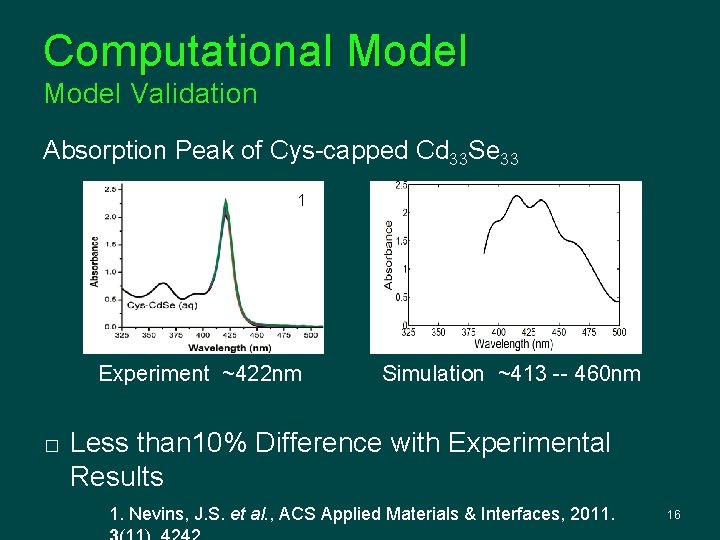
Computational Model Validation Absorption Peak of Cys-capped Cd 33 Se 33 1 Experiment ~422 nm � Simulation ~413 -- 460 nm Less than 10% Difference with Experimental Results 1. Nevins, J. S. et al. , ACS Applied Materials & Interfaces, 2011. 16

Outline Introduction � Computational Model � Results and Discussions � Conclusions and Prospects � 17

Results and Discussions Summary � Magic vs. Non-magic Size QDs � Size Effects of QDs � Ligand Effects on QDs • Bare QDs vs. Passivated QDs • Effects of Length and Function Group (NH 2) • Compare Thiol with Amine and Phosphine 18

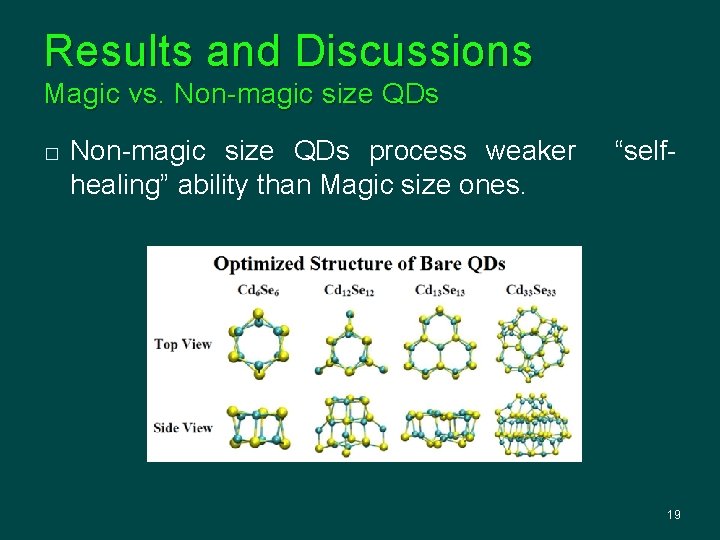
Results and Discussions Magic vs. Non-magic size QDs � Non-magic size QDs process weaker healing” ability than Magic size ones. “self- 19

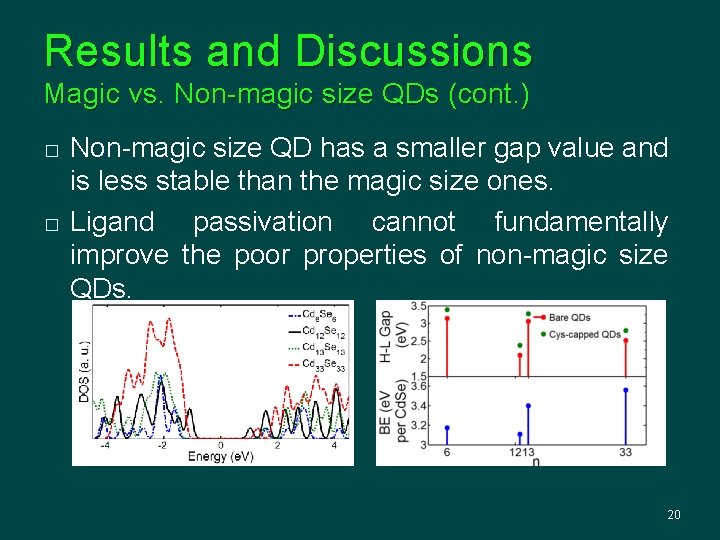
Results and Discussions Magic vs. Non-magic size QDs (cont. ) � � Non-magic size QD has a smaller gap value and is less stable than the magic size ones. Ligand passivation cannot fundamentally improve the poor properties of non-magic size QDs. 20

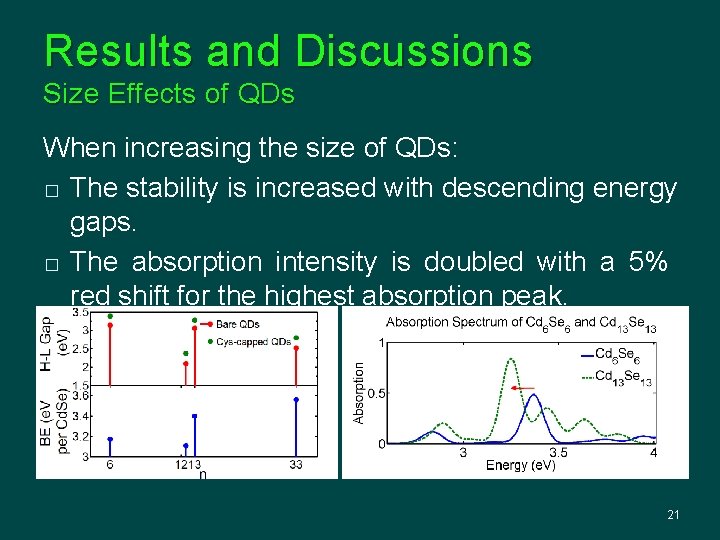
Results and Discussions Size Effects of QDs When increasing the size of QDs: � The stability is increased with descending energy gaps. � The absorption intensity is doubled with a 5% red shift for the highest absorption peak. 21

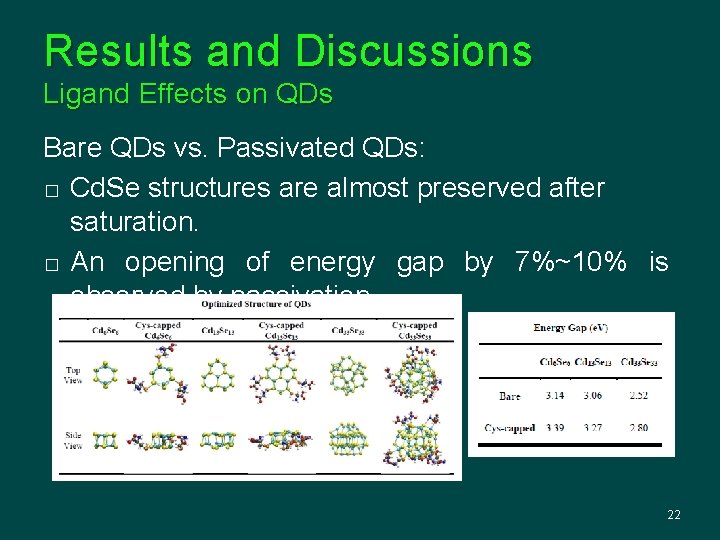
Results and Discussions Ligand Effects on QDs Bare QDs vs. Passivated QDs: � Cd. Se structures are almost preserved after saturation. � An opening of energy gap by 7%~10% is observed by passivation. 22

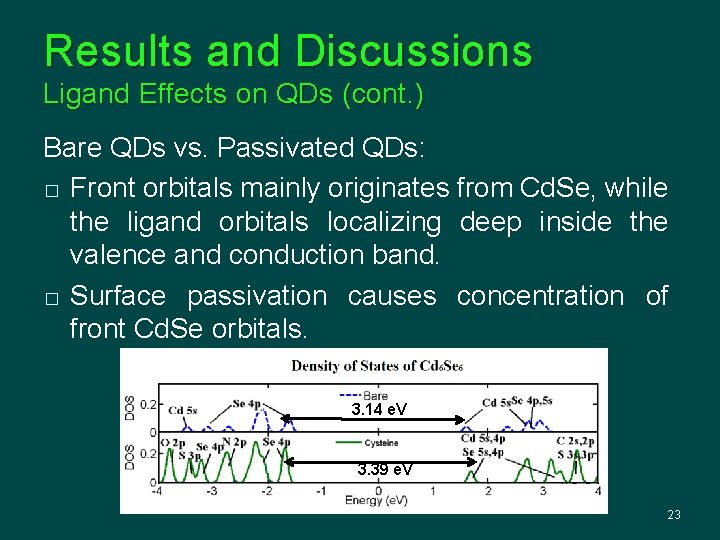
Results and Discussions Ligand Effects on QDs (cont. ) Bare QDs vs. Passivated QDs: � Front orbitals mainly originates from Cd. Se, while the ligand orbitals localizing deep inside the valence and conduction band. � Surface passivation causes concentration of front Cd. Se orbitals. 3. 14 e. V 3. 39 e. V 23

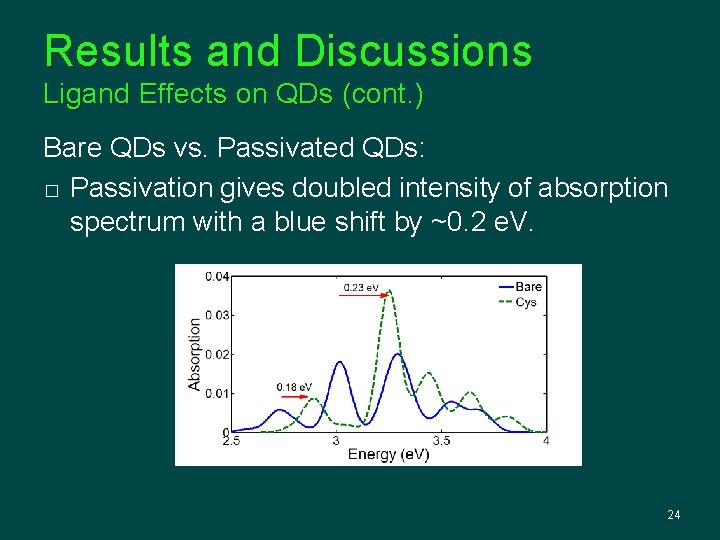
Results and Discussions Ligand Effects on QDs (cont. ) Bare QDs vs. Passivated QDs: � Passivation gives doubled intensity of absorption spectrum with a blue shift by ~0. 2 e. V. 24

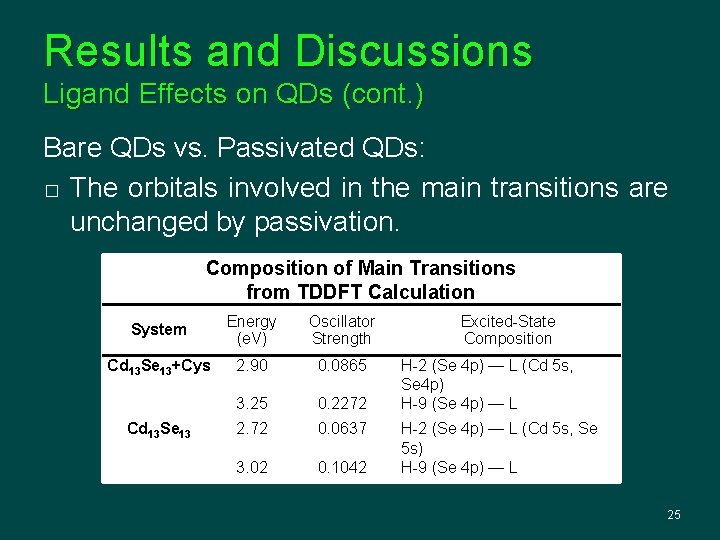
Results and Discussions Ligand Effects on QDs (cont. ) Bare QDs vs. Passivated QDs: � The orbitals involved in the main transitions are unchanged by passivation. Composition of Main Transitions from TDDFT Calculation System Energy (e. V) Oscillator Strength Cd 13 Se 13+Cys 2. 90 0. 0865 3. 25 0. 2272 2. 72 0. 0637 3. 02 0. 1042 Cd 13 Se 13 Excited-State Composition H-2 (Se 4 p) — L (Cd 5 s, Se 4 p) H-9 (Se 4 p) — L H-2 (Se 4 p) — L (Cd 5 s, Se 5 s) H-9 (Se 4 p) — L 25

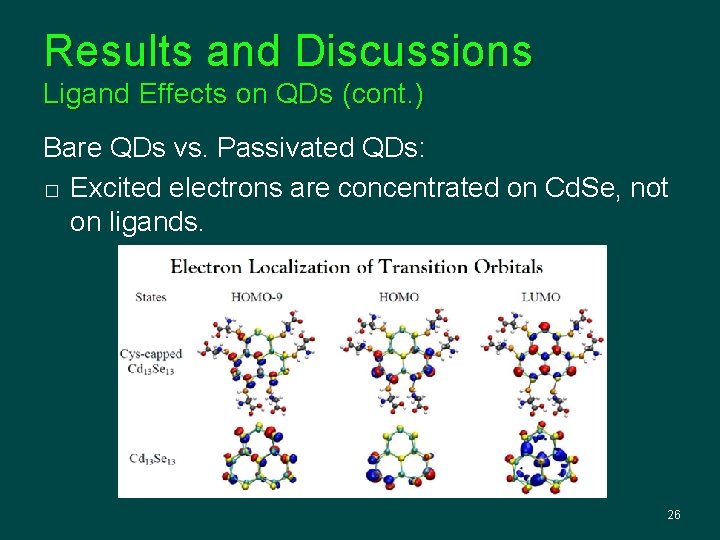
Results and Discussions Ligand Effects on QDs (cont. ) Bare QDs vs. Passivated QDs: � Excited electrons are concentrated on Cd. Se, not on ligands. 26

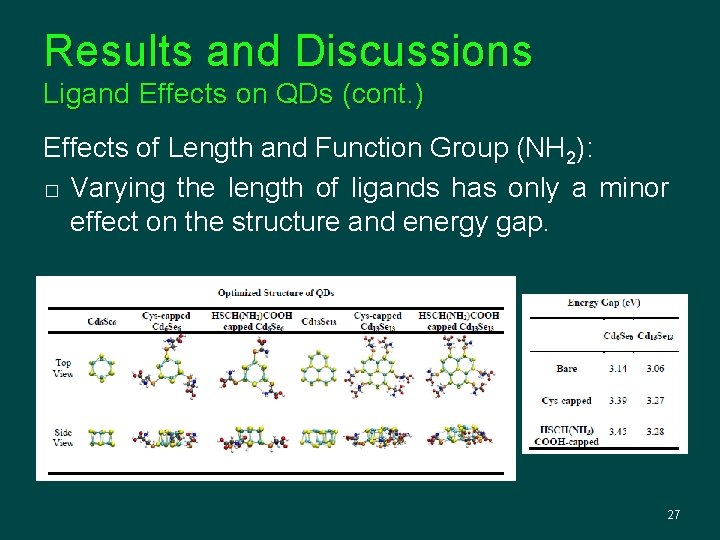
Results and Discussions Ligand Effects on QDs (cont. ) Effects of Length and Function Group (NH 2): � Varying the length of ligands has only a minor effect on the structure and energy gap. 27

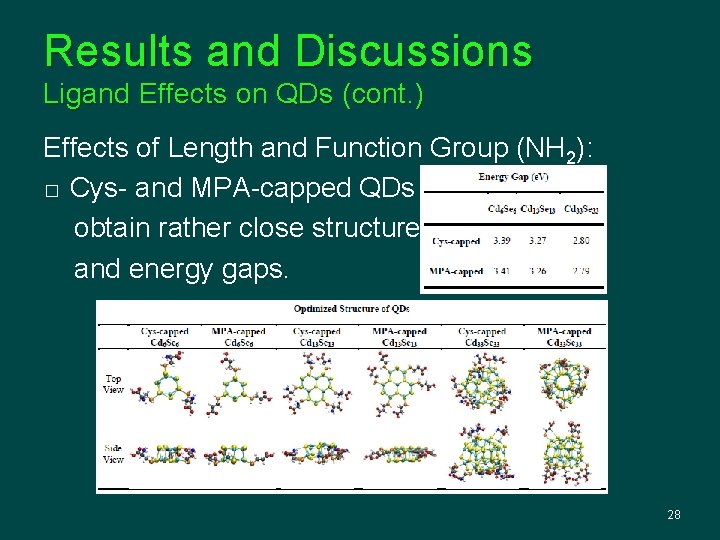
Results and Discussions Ligand Effects on QDs (cont. ) Effects of Length and Function Group (NH 2): � Cys- and MPA-capped QDs obtain rather close structures and energy gaps. 28

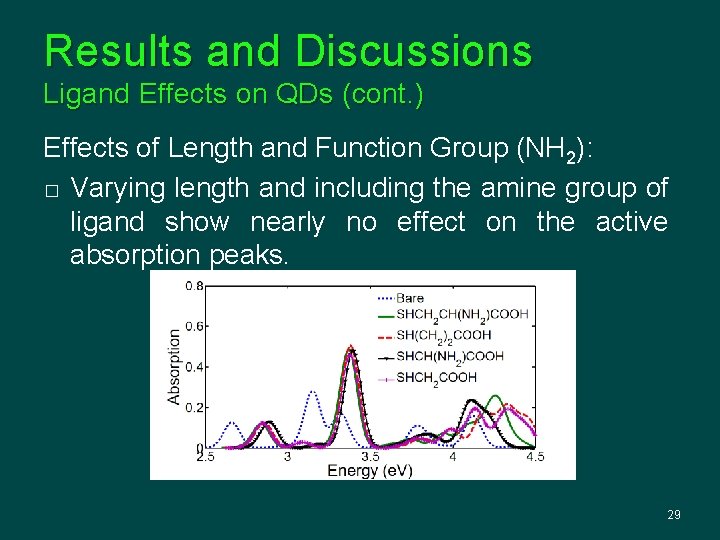
Results and Discussions Ligand Effects on QDs (cont. ) Effects of Length and Function Group (NH 2): � Varying length and including the amine group of ligand show nearly no effect on the active absorption peaks. 29

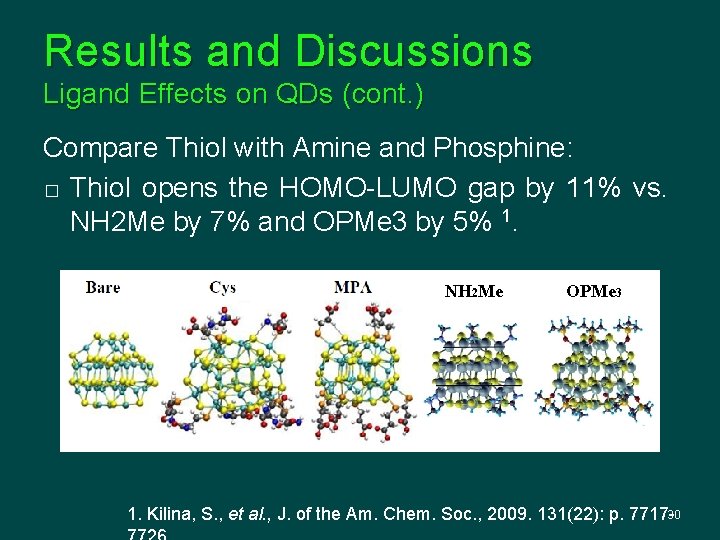
Results and Discussions Ligand Effects on QDs (cont. ) Compare Thiol with Amine and Phosphine: � Thiol opens the HOMO-LUMO gap by 11% vs. NH 2 Me by 7% and OPMe 3 by 5% 1. NH 2 Me OPMe 3 1. Kilina, S. , et al. , J. of the Am. Chem. Soc. , 2009. 131(22): p. 7717 -30

Outline Introduction � Computational Model � Results and Discussions � Conclusions and Prospects � 31

Conclusions and Prospects Conclusions: � Neither “self-healing” nor passivation fundamentally improves the properties. � When increasing the size, the absorption is enhanced with a red shift. � A doubled intensity and a blue shift are observed on the absorption by passivation; Varying length and including the amine group in the thiol have minimal effect; Thiol shows a better ability to improve the band gap opening than amine or phosphine oxide ligands. 32

Conclusions and Prospects: � The effect of ligands as the linker between Cd. Se and Ti. O 2 � The effect of the gold cluster to the Cd. Se-Ti. O 2 devices 33

THANK YOU! 34