A cohort study of mammography screening finds that









- Slides: 16

A cohort study of mammography screening finds that comorbidity measures are insufficient for controlling selection bias. Jonas Czwikla, Klaus Giersiepen, Ingo Langner, Dirk Enders, Franziska Heinze, Heinz Rothgang, Ulrike Haug, Hajo Zeeb, Hans-Werner Hense Journal of Clinical Epidemiology (2018) Presenter: Wen-Ching Lan Date: 2019 / 05 / 15

Introduction l. Randomized controlled trials (RCTs) are the gold standard for the evaluation of screening programs before their nationwide implementation. l. The comparison of all cause mortality between MSP participants and nonparticipants appears useful. l. Statutory Health Insurance (SHI) claims data represent the most comprehensive data source to test this hypothesis in the context of the German MSP. 1

Objective The purpose of this study was to examine the potential of claims-based comorbidity measures for controlling selection bias in observational studies of mammography screening using the single comorbidities considered by the Charlson, Elixhauser, MACSS, and M 3 comorbidity measures. 2

Materials and methods 2. 1. Data source l. This study was based on pseudonymous SHI claims data covering the years from 2007 -2015. l. The data were provided by the BARMER. ldemographic characteristics, insurance periods, inpatient and outpatient diagnoses, as well as outpatient therapeutic and diagnostic procedures were available. l. ICD-10 -GM l. Outpatient procedures were coded according to the doctor’s fee scale (Einheitlicher Bewertungsmaßstab [EBM]). 3

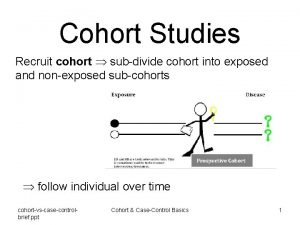
Materials and methods 2. 2. Study design and population l. Retrospective cohort study (over a 2 -year period covering the invitation years 2010 and 2011. ) l. Women with the specific EBM code 01750→ MSP participants l. Main analysis (cohort I) 1. Age 50 -68 years in 2010 2. Continuous insurance between January 1, 2007 and December 31, 2011. 3. No inpatient or outpatient : ICD-10 -GM C 50 or ICD-10 -GM Z 85. 3 in 2007, 2008 or 2009. 4

Materials and methods l. Start date set at January 1, 2012. l. End date (1)death, (2)end of continuous insurance, (3)December 31, 2015 l. Sensitivity analysis using an index date approach (cohort II) 2007 -2009 5

Materials and methods 2. 3. Identification of comorbidity l. Based on main and secondary hospital discharge diagnoses. l. Cohort 1 (1)2010 -2011, (2) 2009, (3) 2008 -2009, (4) 2007 -2009, (5)2007 -2011. l. Cohort 2 (6) 1 year before cohort entry, (7) 2 years before cohort entry, (8) 3 years before cohort entry. 6

Materials and methods 2. 4. Statistical analysis 1. Cox proportional hazards regression 1) Crude 2) Adjusted age in 2010 and federal state of residence 3) Charlson, Elixhauser, MACSS, or M 3 comorbidity measure 2. Propensity score estimates were used for a Greedy 5→ 1 Digit Matching 1: 1 l. SAS Enterprise Guide 7. 1. 7

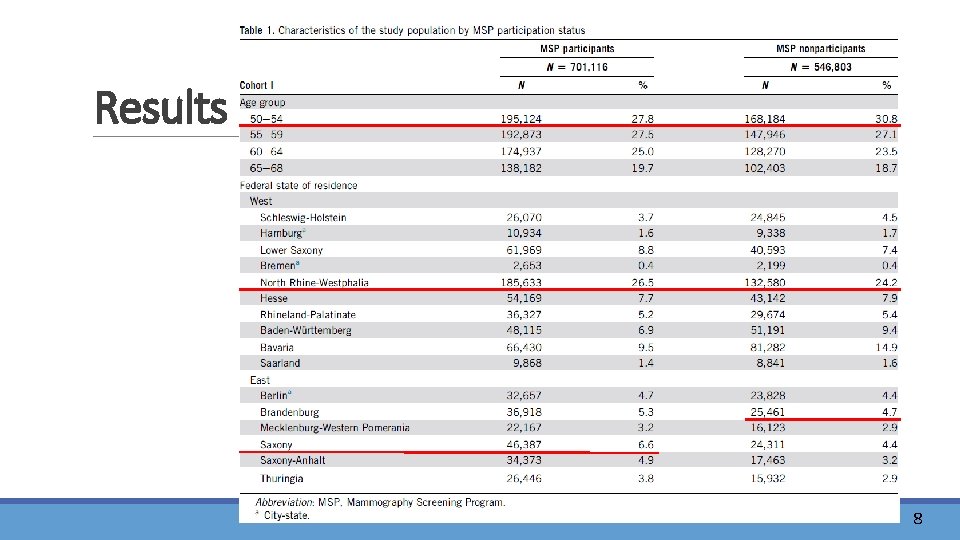
Results 8

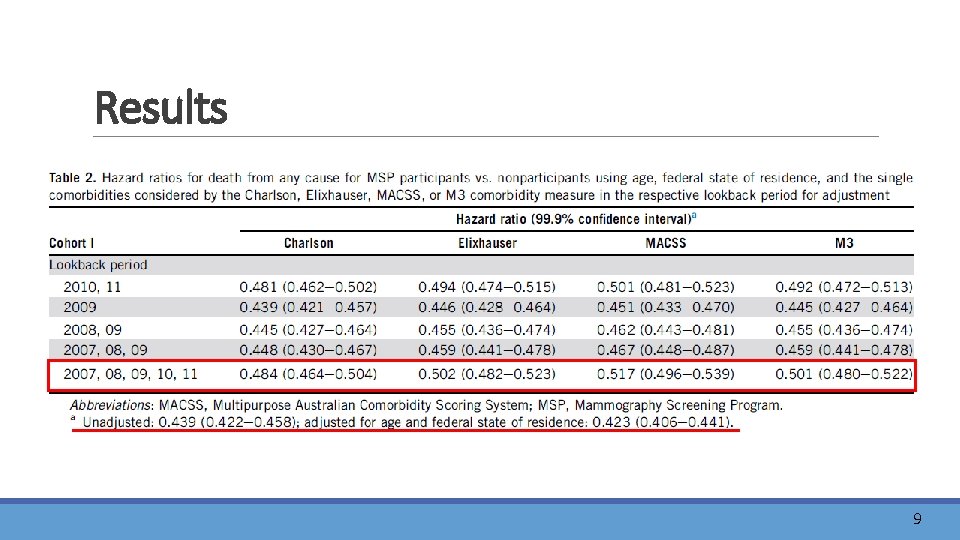
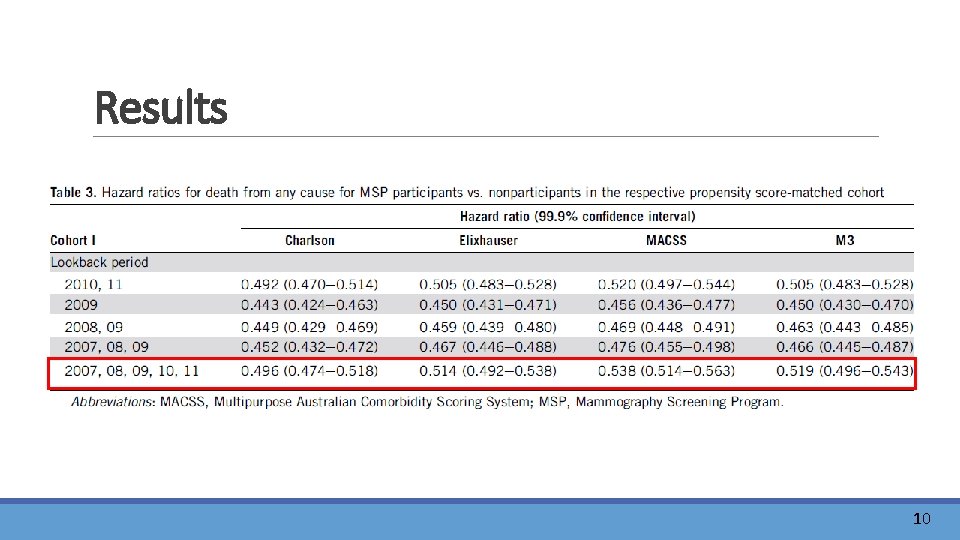
Results 9

Results 10

Discussion l. MSP may reduce breast cancer mortality by about 20% , one would theoretically expect allcause mortality in this population to be decreased by 1. 8%. l. After adjustment for age, federal state of residence and the single comorbidities considered by the Charlson, Elixhauser, MACSS, or M 3 comorbidity measure, participants were still at least 48% less likely to die, suggesting an insufficient control of selection bias. lhealthy volunteer bias 11

Strengths 1. Two established and two new claimsbased comorbidity measures to examine 2. comprised 9 years of longitudinal data for more than 1. 24 million continuously insured women eligible for MSP participation, allowing precise estimates of selection bias. 12

limitations 1. German SHI claims data contain no information on the specific cause of death. 2. To examine selection bias, we used rather short follow-up periods. 3. MSP invitation dates are not available in Germany. 13

Conclusions Mammography screening participants and nonparticipants are not comparable in terms of their health status. It is not sufficient to use the single comorbidities considered by the Charlson, Elixhauser, MACSS, or M 3 comorbidity measure for controlling selection bias in observational studies of mammography screening. 14

Thanks for listening.
 Retrospective cohort study
Retrospective cohort study He who finds a wife finds a good thing
He who finds a wife finds a good thing Ecological study vs cohort study
Ecological study vs cohort study Difference between case control and cohort study
Difference between case control and cohort study Cohort study example
Cohort study example Retrospective cohort study
Retrospective cohort study Bias in cohort study
Bias in cohort study Cohort stidy
Cohort stidy Cohort study community medicine
Cohort study community medicine Cohort study definition
Cohort study definition Retrospective cohort study vs case control
Retrospective cohort study vs case control Difference between case control and cohort study
Difference between case control and cohort study Retrospective cohort study vs case control
Retrospective cohort study vs case control Mqsa requirements for mammography checklist
Mqsa requirements for mammography checklist Mammography mcqs
Mammography mcqs Ohiohealth berger hospital mammography circleville
Ohiohealth berger hospital mammography circleville Breast exam
Breast exam