58 Earth System Science 58 Earth System Science



























- Slides: 46

58 Earth System Science

58 Earth System Science • Introduction • Earth’s System Has Four Compartments • Biogeochemical Cycles, Water, and Fire • The Carbon Cycle • The Nitrogen Cycle • The Sulfur Cycle • The Phosphorus Cycle • Interactions among Biogeochemical Cycles • Visions of the Future

58 Introduction • Earth system science is a new field of inquiry focusing on Earth as a whole. • A system is a group of entities that interact to yield a product. • An example of a system is the organs that work together to support the existence of an animal. • Earth’s system is composed of cycles of materials, inputs of solar energy, and interactions between living organisms and the physical environment.

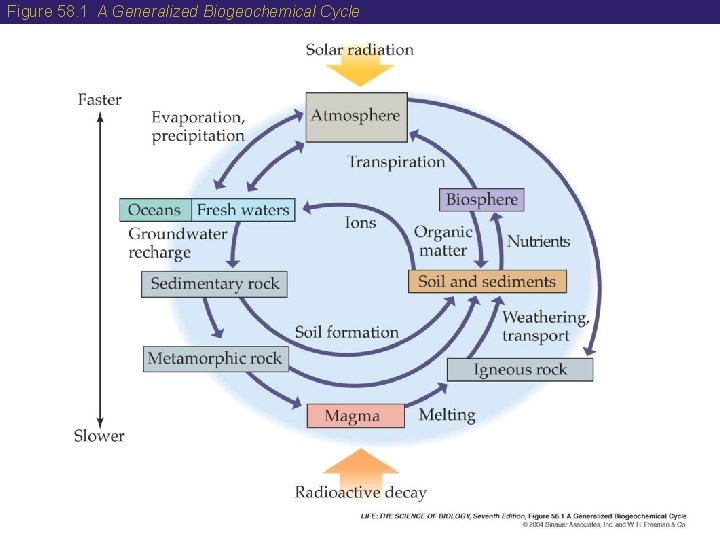
58 Earth’s System Has Four Compartments • Earth is essentially a closed system with respect to atomic matter, but an open system with respect to energy (solar). • Energy from the sun combined with energy from radioactive decay drives the processes that move materials around the planet. • Many of these processes are cyclic. • The cycling of elements can be understood by dividing the physical environment into four interacting compartments: oceans, fresh waters, atmosphere, and land.

Figure 58. 1 A Generalized Biogeochemical Cycle

58 Earth’s System Has Four Compartments • Earth has many unusual properties, including the presence of life, an ocean, moderate surface temperature, continental drift, and a large moon. • The moon stabilized the tilt of Earth on its axis, produces ocean tides, and slows Earth’s rotation.

58 Earth’s System Has Four Compartments • Oceans receive materials from the land in runoff from rivers. • Most materials that cycle through the four compartments end up in oceans. • Except near land on continental shelves, ocean water mixes slowly and most elements sink to the sea floor. • Concentration of mineral nutrients is low in most ocean waters.

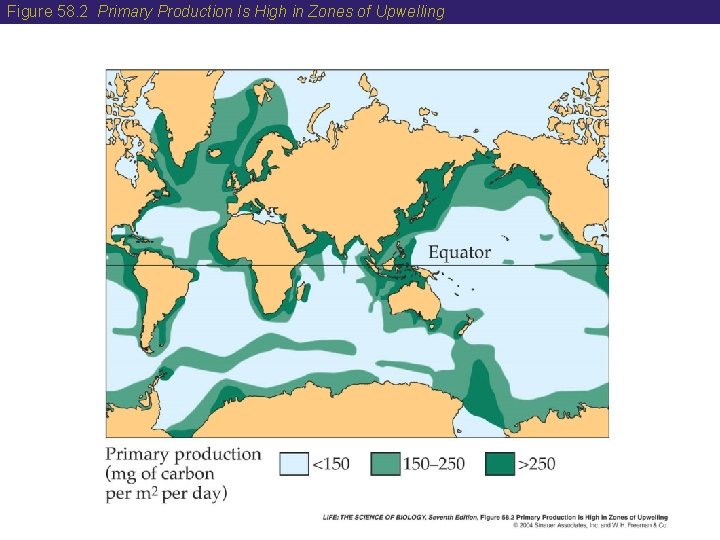
58 Earth’s System Has Four Compartments • Some elements are brought back to the surface at zones of upwelling. • Upwelling occurs when offshore winds push surface waters away from shore, causing cold, nutrient-rich bottom waters to rise to the surface. • Most of the world’s great fisheries are in these zones.

Figure 58. 2 Primary Production Is High in Zones of Upwelling

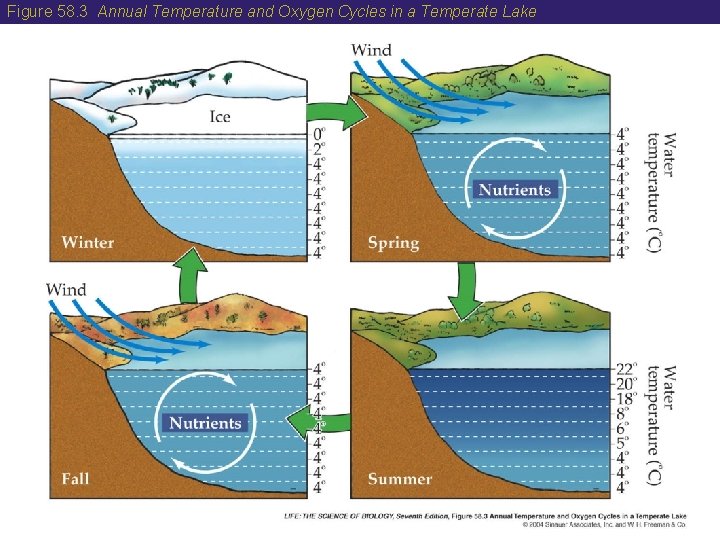
58 Earth’s System Has Four Compartments • Only a small fraction of Earth’s water resides in lakes and rivers at any certain time. • Most mineral nutrients enter the freshwater compartment from weathering of rocks and enter rivers through surface flow or ground water. • After entering rivers, mineral nutrients are rapidly carried to lakes or oceans. • In lakes, nutrients are taken up by organisms, which eventually die and sink to the bottom. • Decomposition of dead organisms consumes the oxygen in the bottom water of the lake.

58 Earth’s System Has Four Compartments • The surface water of lakes therefore becomes rapidly depleted of nutrients but is high in oxygen, while the bottom water is high in nutrients but low in oxygen. • A process called turnover mixes the water in lakes, resulting in a more even distribution of oxygen and nutrients. • Turnover results from wind and from temperature differences in the lake. Water is most dense at 4°C.

Figure 58. 3 Annual Temperature and Oxygen Cycles in a Temperate Lake

58 Earth’s System Has Four Compartments • The atmosphere is a thin layer of gases surrounding Earth. • About 80 percent of the mass is in the lowest layer, the troposphere. • Most global air circulation and atmospheric water vapor are confined to the troposphere. • The stratosphere extends from the top of the troposphere to about 50 km above Earth’s surface. • Materials tend to stay in the stratosphere for a relatively long time. • Ozone in the stratosphere absorbs short-wave ultraviolet light.

58 Earth’s System Has Four Compartments • The atmosphere is approximately 78% N 2, 21% O 2, 1% argon, and 0. 03% CO 2, plus other trace gases such as xenon, helium, and ozone. • The greenhouse gases of the atmosphere (water vapor, CO 2, and O 3 and others) trap heat that Earth radiates back to space. • Without an atmosphere, the average surface temperature of Earth would be about – 18°C, rather than its actual +17°C.

58 Earth’s System Has Four Compartments • Most land above sea level is in the Northern Hemisphere. • Regional and local deficiencies of particular elements strongly affect ecosystem processes on land. • The land compartment is connected to the atmosphere by organisms that utilize chemicals from the air and contribute chemicals to it. • Human life depends on soil fertility and the productivity of terrestrial ecosystems.

58 Biogeochemical Cycles, Water, and Fire • Carbon, hydrogen, oxygen, nitrogen, phosphorus, and sulfur are the elements organisms need in large quantities. • The patterns of movement of chemical elements through organisms and the four compartments of the physical environment are called biogeochemical cycles.

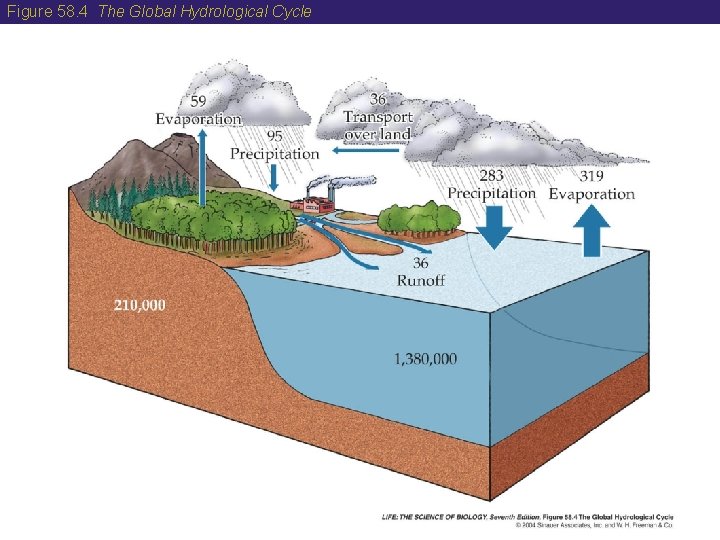
58 Biogeochemical Cycles, Water, and Fire • The cycling of water through the oceans, atmosphere, fresh waters, and land is known as the hydrological cycle. • The hydrological cycle operates because more water is evaporated from the oceans than is returned to them. • The excess falls as precipitation over land. • More water is deposited on land than is evaporated from it, therefore, excess water is returned to the oceans by rivers, coastal runoff, and groundwater flows.

Figure 58. 4 The Global Hydrological Cycle

58 Biogeochemical Cycles, Water, and Fire • Earth’s 16 largest rivers account for more than one-third of total water discharge to the oceans. • Dams, canals, and reservoirs have major effects on the temporal and spatial distribution of fresh water on Earth. • More water now evaporates from land less flows to oceans than before the Industrial Revolution. • Fresh water flow patterns are also being altered. • Damming of the Columbia River, for example, has altered the seasonal variation of discharge by the river and threatened the survival of the salmon population.

58 Biogeochemical Cycles, Water, and Fire • In some areas, groundwater is being seriously depleted because humans are using it more rapidly for irrigation purposes than it can be replaced. • According to a study published in 2000, if water consumption patterns continue, by 2025 almost one-half of the world’s population (primarily in Asia) will live in areas with inadequate water supplies.

58 Biogeochemical Cycles, Water, and Fire • Fires consume the energy of, and release chemical elements from, the vegetation they burn. • Some nutrients, such as nitrogen, sulfur, and selenium, are easily vaporized by fire and returned to the groundwater by rain falling on the burned area. • Fires release large amounts of carbon into the atmosphere and produce significant quantities of greenhouse gases.

58 The Carbon Cycle • All living organisms must have access to carbon atoms to survive. • Nearly all the carbon in organisms comes from CO 2 in the atmosphere or HCO 3– in water. • Autotrophs incorporate carbon into organic molecules by photosynthesis. • All heterotrophs get carbon by consuming autotrophs or other heterotrophs, their remains, or their waste products. • Photosynthesis removes carbon from the atmosphere; respiration returns carbon to the atmosphere.

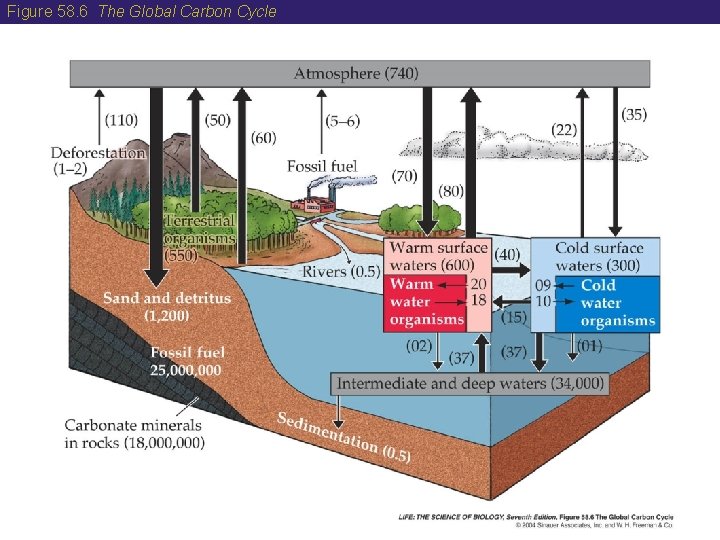
Figure 58. 6 The Global Carbon Cycle

58 The Carbon Cycle • Most of Earth’s carbon is stored in the oceans. • During the remote past, great quantities of carbon were removed from the carbon cycle when large numbers of organisms died in anaerobic environments and the organic material was converted to fossil fuels such as oil, natural gas, coal, and peat. • The concentration of CO 2 in the atmosphere is increasing due to human burning of these fossil fuels.

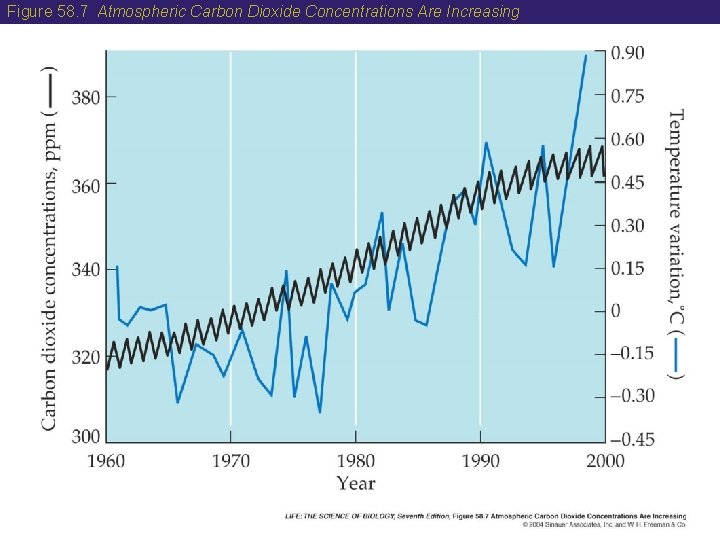
Figure 58. 7 Atmospheric Carbon Dioxide Concentrations Are Increasing

58 The Carbon Cycle • Increased levels of atmospheric CO 2 have resulted in an increase in Earth’s average temperature. • Computer models of Earth’s system indicate that if atmospheric CO 2 doubles, climates will be shifted toward the poles and sea level changes due to melting ice caps will cause flooding in coastal areas. • The adverse effects of increased CO 2 levels in the atmosphere are likely to be severe. • Scientists are studying methods to reduce CO 2 release into the atmosphere.

58 The Carbon Cycle • The oceans determine atmospheric CO 2 concentrations. • Two factors that affect the rate of CO 2 movement from the atmosphere to the oceans are photosynthesis by plankton and formation of calcium carbonate shells by marine organisms. • The rate of CO 2 movement to deep ocean waters depends on the ocean conveyor belt, which results from the sinking of dense, saline water in the North Atlantic Ocean. • The ocean conveyer belt may weaken if melting of the Greenland ice cap discharges great quantities of water into the ocean.

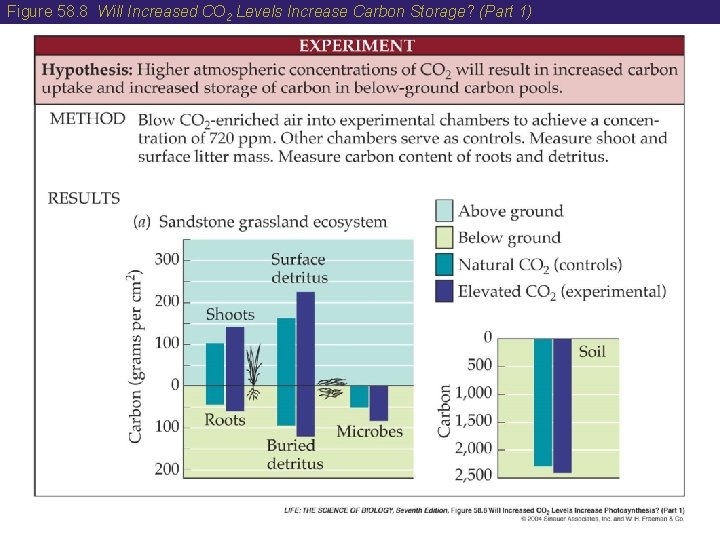
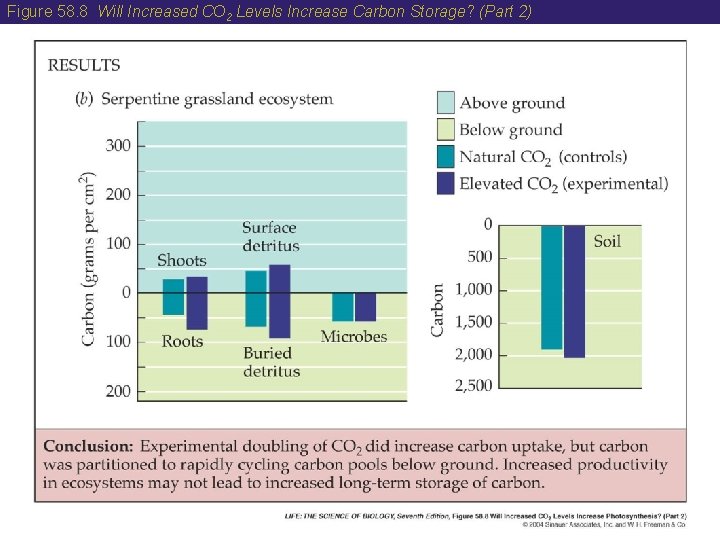
58 The Carbon Cycle • Consumption of CO 2 in photosynthesis by terrestrial vegetation appears to exceed the production of CO 2 by respiration. Therefore, Earth’s forests are storing carbon. • Ecologists are conducting experiments to determine the effect of higher CO 2 levels on photosynthesis and carbon storage.

Figure 58. 8 Will Increased CO 2 Levels Increase Carbon Storage? (Part 1)

Figure 58. 8 Will Increased CO 2 Levels Increase Carbon Storage? (Part 2)

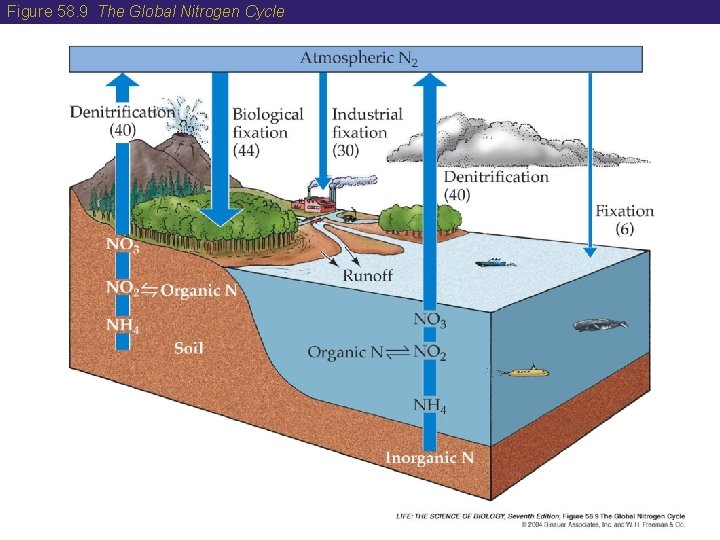
58 The Nitrogen Cycle • Nitrogen gas (N 2) makes up 78 percent of the Earth’s atmosphere but most organisms cannot use nitrogen in this form. • A few species of microorganisms can fix nitrogen: that is, convert atmospheric nitrogen to forms useable by plants. • Microorganisms also facilitate denitrification, the return of nitrogen to the atmosphere. • The movement of nitrogen on Earth is called the global nitrogen cycle. • Humans have altered the nitrogen cycle by using nitrogen-based fertilizers and burning fossil fuels (which produces nitric oxide).

Figure 58. 9 The Global Nitrogen Cycle

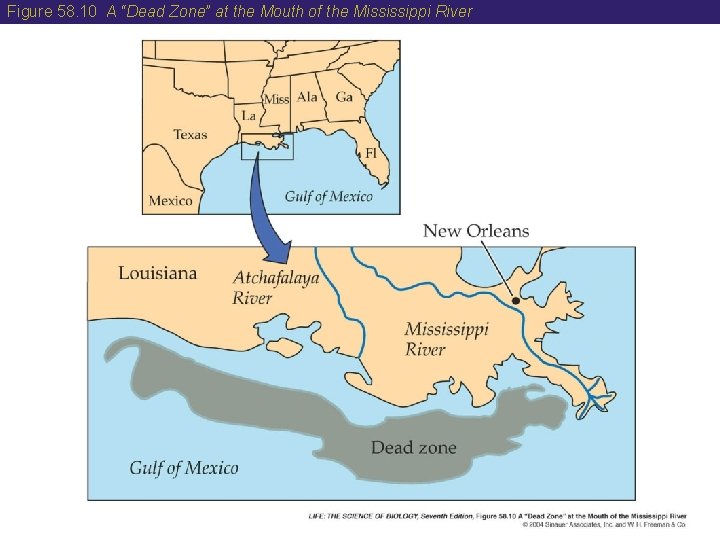
58 The Nitrogen Cycle • Negative effects of changes in the nitrogen cycle include the contamination of groundwater by nitrate and increases in atmospheric nitrous oxide and tropospheric ozone. • Excess nitrogen from fertilization runs off into rivers, lakes, and eventually the ocean. • The addition of nutrients to water bodies, called eutrophication, can have negative effects such as the “dead zone” in the Gulf of Mexico at the Mississippi River Delta.

Figure 58. 10 A “Dead Zone” at the Mouth of the Mississippi River

58 The Sulfur Cycle • Sulfur is apparently always abundant enough to meet the needs of living organisms. • Volcanoes and fumaroles emit sulfur dioxide (SO 2) and hydrogen sulfide (H 2 S). These are the natural nonbiological fluxes of sulfur, but they are rare events. • Certain marine algae produce dimethyl sulfide (CH 3 SCH 3), which accounts for half of the biotic component of the sulfur cycle. • Sulfur plays an important role in global climate. • Dimethyl sulfide is the major component of particles in the air, which allow clouds to form.

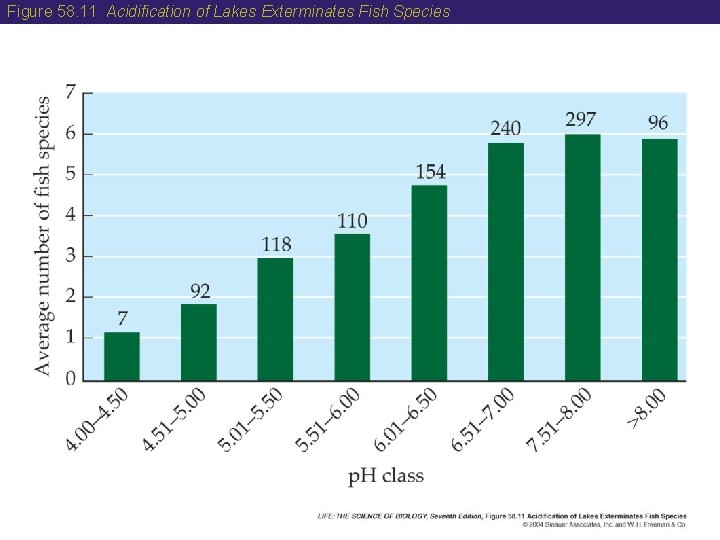
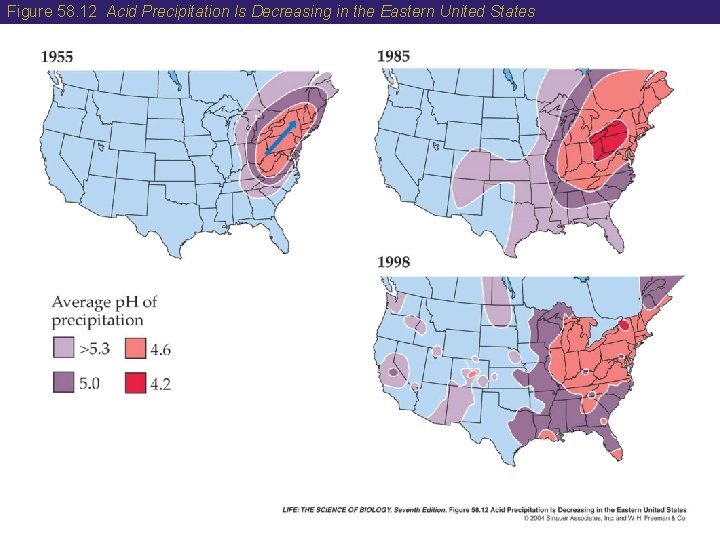
58 The Sulfur Cycle • Humans have altered the sulfur cycle by burning fossil fuels. • Acid precipitation is caused by sulfuric and nitric acids derived largely from the burning of fossil fuels. • Acidification of lakes in the Adirondack region of New York has reduced fish species richness there. • The Clean Air Act Amendments of 1990 have helped reduce acid precipitation in the Eastern United States. • Canadian ecologists have shown that lakes can recover from acid conditions if the amount of sulfuric acid is reduced.

Figure 58. 11 Acidification of Lakes Exterminates Fish Species

Figure 58. 12 Acid Precipitation Is Decreasing in the Eastern United States

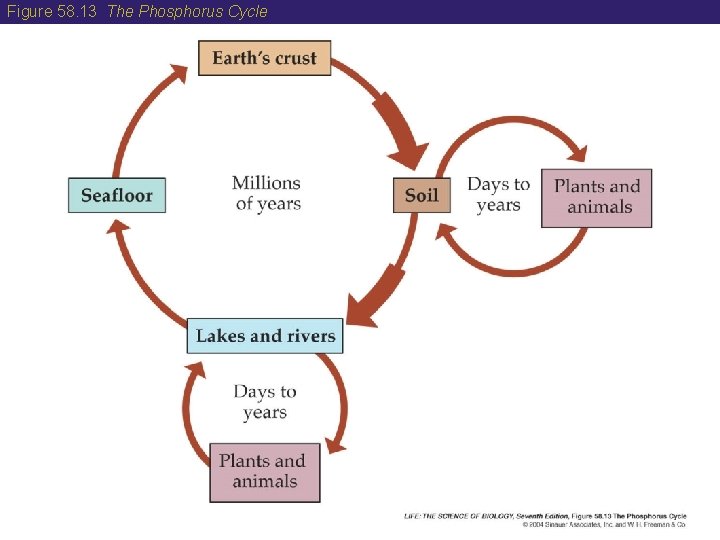
58 The Phosphorus Cycle • Phosphorus, a key component of DNA and ATP, is essential for life. • Phosphorus does not have a gaseous phase like the other elements. • The global phosphorus cycle is very slow (taking millions of years to complete) because the processes of rock formation on the ocean bottom, subsequent uplifting, and weathering of rock into soil all occur slowly. • However, a single atom of phosphorus may cycle rapidly through organisms.

Figure 58. 13 The Phosphorus Cycle

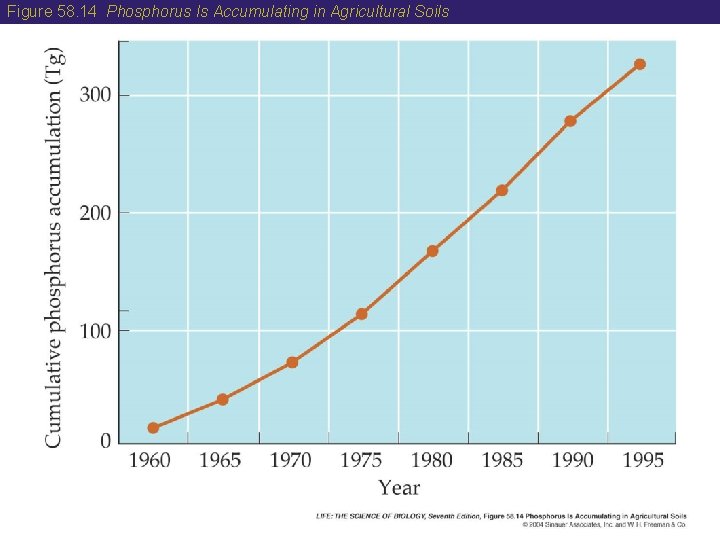
58 The Phosphorus Cycle • Human activity has affected the phosphorus cycle. About 90 percent of the phosphorus that is mined is used to produce fertilizers and animal feeds. • Phosphorus is accumulating in soils at a rapid rate due to fertilizer use. • Eutrophication of lakes with phosphorus allows algae and bacteria to multiply, forming blooms. • Decomposition of the dead cells occurs after the bloom consumes all oxygen in the lake and anaerobic bacteria take over.

Figure 58. 14 Phosphorus Is Accumulating in Agricultural Soils

58 The Phosphorus Cycle • Lake Erie is a eutrophic lake today, although improved municipal waste handling has reduced the phosphorus level in the lake by 80% from its maximum level. • The potential for recovery and recycling of phosphorus is high. The amount of phosphorus contained in sewage and animal wastes could supply major industrial needs. • Careful application of fertilizers on agricultural lands can reduce the rate of phosphorus accumulation without reducing crop yields.

58 Interactions among Biogeochemical Cycles • Biogeochemical cycles are strongly interrelated. Humans have altered biogeochemical cycles on Earth. • Winter typically kills 99 percent of pathogenic organisms. However, global warming is causing warmer winter conditions, which can lead to increased outbreaks of disease. • The 1996 assessment report of the Intergovernmental Panel on Climate Change expressed concern about the effects of climate change on human health.

58 Visions of the Future • Human population growth has slowed since 1965 due to voluntary reductions in fertility. • If humans are to live on Earth in a sustainable manner, major changes in our use of environmental resources will be necessary.

Figure 58. 15 Earth from Space