5 National and international reporting of adverse events










- Slides: 20

5. National and international reporting of adverse events: mechanisms, routes and resources Multi-partner training package on active TB drug safety monitoring and management (a. DSM) July 2016

Objectives of the presentation By the end of this presentation, the participant is expected to be able to: 1. describe the mechanisms for adverse event reporting within the country 2. describe the mechanism for adverse event reporting to the global a. DSM database 3. identify the resources needed to ensure that the whole system works

Core package of a. DSM basic principles • all serious adverse events (SAEs) detected need to be monitored • however, all adverse events (AEs) detected need to be clinically managed, regardless of seriousness • treatment sites with additional resources may also monitor other AEs that are of clinical significance or of special interest to the programme

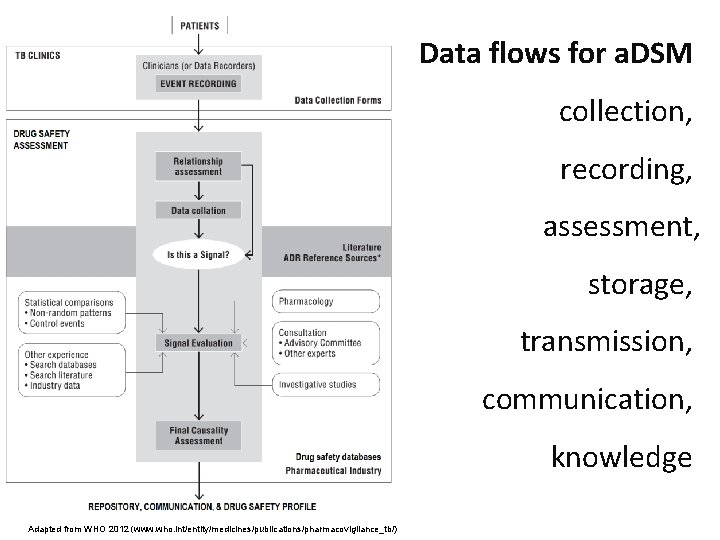
Data flows for a. DSM collection, recording, assessment, storage, transmission, communication, knowledge Adapted from WHO 2012 (www. who. int/entity/medicines/publications/pharmacovigilance_tb/)

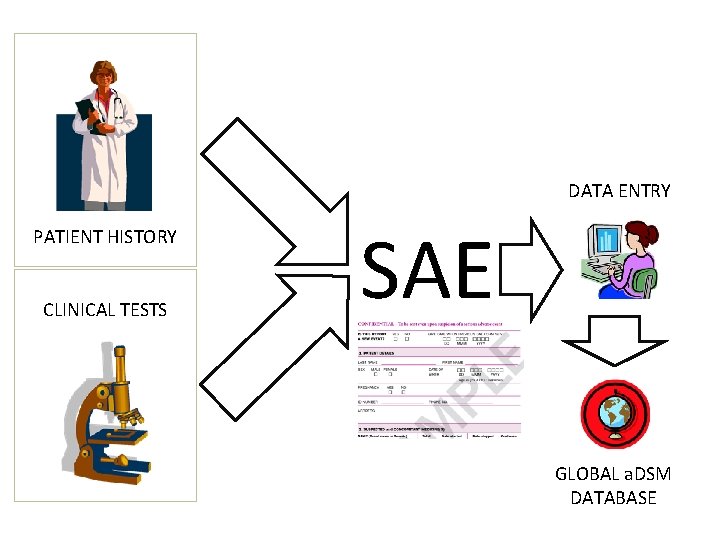
DATA ENTRY PATIENT HISTORY CLINICAL TESTS SAE GLOBAL a. DSM DATABASE

Data collection create data collection tools and database • • Forms shown here are examples: national programmes can develop and adapt their own forms (electronic or paper) When revising data collection tools and systems, programmes need to : – Ensure where possible that new data elements needed for a. DSM are built into existing system rather than creating new databases afresh, avoiding parallel systems, simplifying as much as possible – Strive for interoperability with other registries (e. g. TB patients’ database, laboratory information system) for any new system created – Apply usual “healthy practices” in data entry & transfer


UNION multi-centre project MSF centres (Uzbekistan, Swaziland)

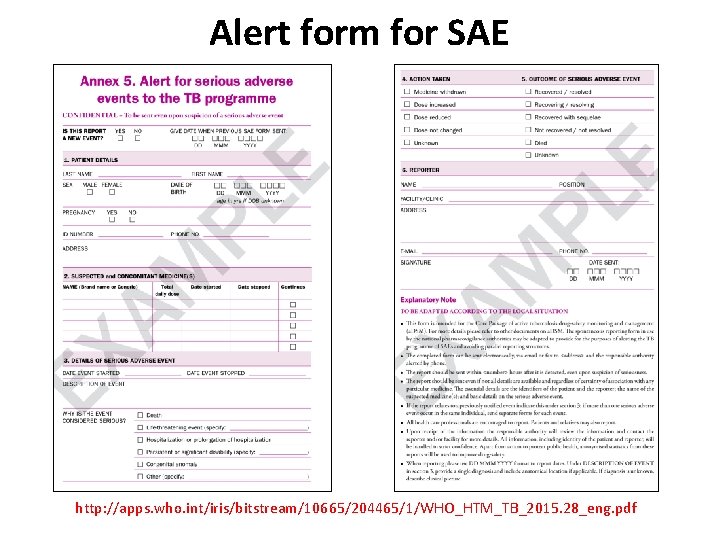
Alert form for SAE http: //apps. who. int/iris/bitstream/10665/204465/1/WHO_HTM_TB_2015. 28_eng. pdf

Data collection form - Initiation p e l S m a

Data collection form - Review p e l S m a

a. DSM Report Forms • There are 3 instances at which data are expected to be collected and reported: 1. Alert of a suspected/confirmed SAE 2. Initial detailed report following the alert SAE 3. Review detailed report following initial report • TB programmes may develop separate forms (paper or electronic) for the reporting at these three steps. Otherwise they may adapt, e. g; Initial detailed report doubles as an alert form, with full details on this form completed later, after the alert

Assigning severity (1) • Simplest is a scale from mild->moderate->severe as determined by the clinician and/or patient • Severity scales are available (e. g. DAIDS, CTCAE, ANRS…). They are useful to classify results of laboratory and other special tests • Tor remember that these scales have been developed for conditions other than TB treatment (AIDS, cancer…) • They often combine elements of seriousness with severity

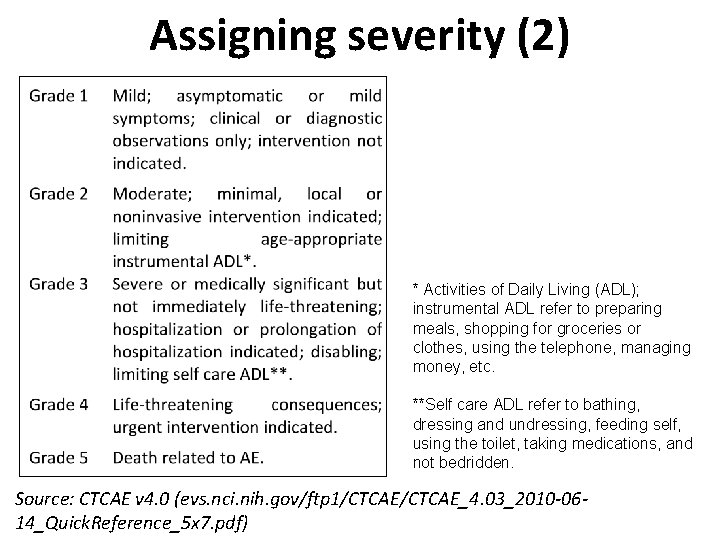
Assigning severity (2) * Activities of Daily Living (ADL); instrumental ADL refer to preparing meals, shopping for groceries or clothes, using the telephone, managing money, etc. **Self care ADL refer to bathing, dressing and undressing, feeding self, using the toilet, taking medications, and not bedridden. Source: CTCAE v 4. 0 (evs. nci. nih. gov/ftp 1/CTCAE_4. 03_2010 -0614_Quick. Reference_5 x 7. pdf)

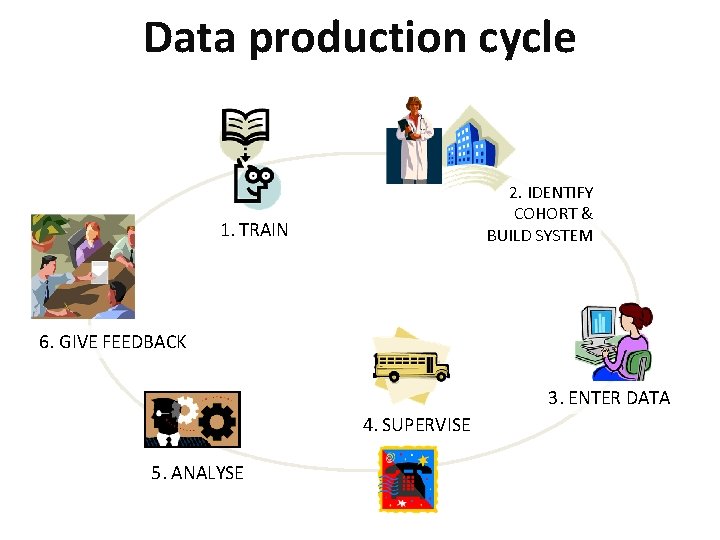
Data production cycle 2. IDENTIFY COHORT & BUILD SYSTEM 1. TRAIN 6. GIVE FEEDBACK 3. ENTER DATA 4. SUPERVISE 5. ANALYSE


Resources for reporting • Its proper functioning will depend on the mobilization of resources (funding, equipment, consumables, human resource capacity) • Technical (KNCV, MSF, MSH, WHO, TDR…) and funding agencies (GF, USAID…) can support activities to put in place the required elements – training, development of normative tools, information systems • In the long run, a. DSM needs to become part of the routine monitoring and patient care structure of a TB treatment programme and necessary resources made available through domestic and external funds

Global a. DSM database • A global a. DSM database was created in July 2016 • It will be coordinated by the Special Programme for Research and Training in Tropical Diseases at WHO Headquarters (TDR) and the WHO/GTB • Physically housed in Luxembourg Institute of Health (LIH), which will be responsible for its day-to-day management • Countries can report SAEs and other AEs to the database, and the reporting may come from patients treated with medicines which are new or repurposed for an indication on TB. (more details in modules 11 iv and 11 vi in this series)

WHO/HTM/TB/2011. 22 whqlibdoc. who. int/publications/2012/9789241564465_eng. pdf

Conclusion • The backbone of AE monitoring and management relies heavily on the proper functioning of a properly-resourced system to collect and manage the data • Upstream of the data collection process are a series of enabling activities, including the establishment of a regulatory framework, the human resource element (roles, responsibilities, training), and the case definitions for reporting • The routing of data needs to proceed uninterruptedly from the site where the event happens, to the national dataset, up to the international level • The process is also subject to quality checks at national and international level. Causality assessment and process indicators need to be done within the country; signal detection is a process best done at international level.
 Novartis adverse event reporting
Novartis adverse event reporting Ahca adverse incident reporting
Ahca adverse incident reporting Adverse events in hospital
Adverse events in hospital Ir adverse event
Ir adverse event Puerperal endometritis
Puerperal endometritis Adverse events following immunization (aefi) course answers
Adverse events following immunization (aefi) course answers Events after the reporting date
Events after the reporting date Mutually exclusive vs non mutually exclusive
Mutually exclusive vs non mutually exclusive National mechanism for reporting and follow-up
National mechanism for reporting and follow-up National and kapodistrian university of athens events
National and kapodistrian university of athens events International financial reporting standards 9
International financial reporting standards 9 National deworming day
National deworming day Ndd reporting format 2021
Ndd reporting format 2021 National reporting system
National reporting system Deworming poster images
Deworming poster images National ombudsman reporting system
National ombudsman reporting system National ombudsman reporting system
National ombudsman reporting system Exceptional control flow
Exceptional control flow Local national and international issues
Local national and international issues Need of national integration
Need of national integration Adverse reaction definition
Adverse reaction definition






































