4 2 Continued Writing Net Ionic Equations Learning





- Slides: 11

4. 2 Continued… Writing Net Ionic Equations Learning Goals: 1. To dissociate all products and reactants (when applicable) 2. To determine the state of reactants and products using solubility rules. 3. To determine spectator ions present in solution

Writing ionic equations n n Dissociation- to break apart into positive and negative ions A soluble compound will dissociate into ions. An insoluble compound does not dissociate. Precipitate- insoluble solid formed in a reaction. It looks cloudy.


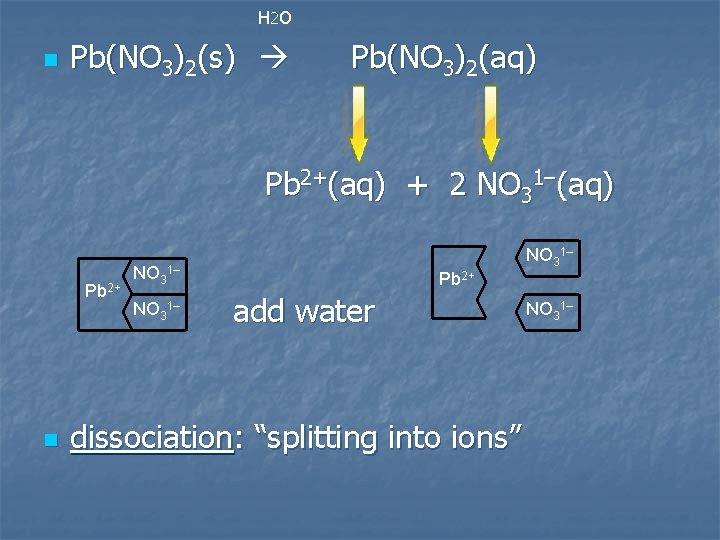
H 2 O n Pb(NO 3)2(s) Pb(NO 3)2(aq) Pb 2+(aq) + 2 NO 31–(aq) Pb 2+ n NO 31– Pb 2+ add water dissociation: “splitting into ions” NO 31–

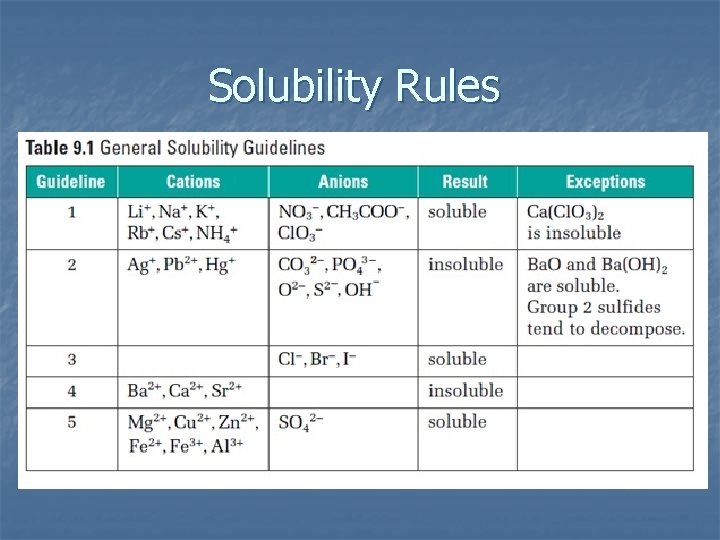
Solubility Rules

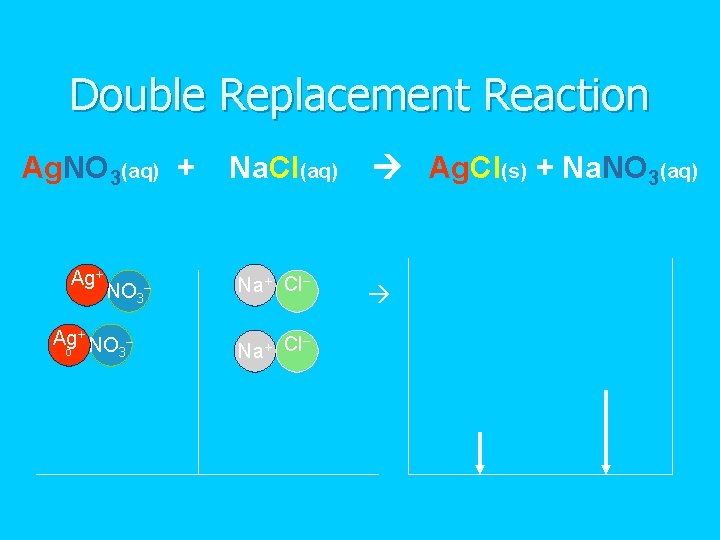
Double Replacement Reaction Ag. NO 3(aq) + Ag+ NO 3– Ag+ NO o 3 – Na. Cl(aq) Na+ Cl– – Na+ Cl Ag. Cl(s) + Na. NO 3(aq)

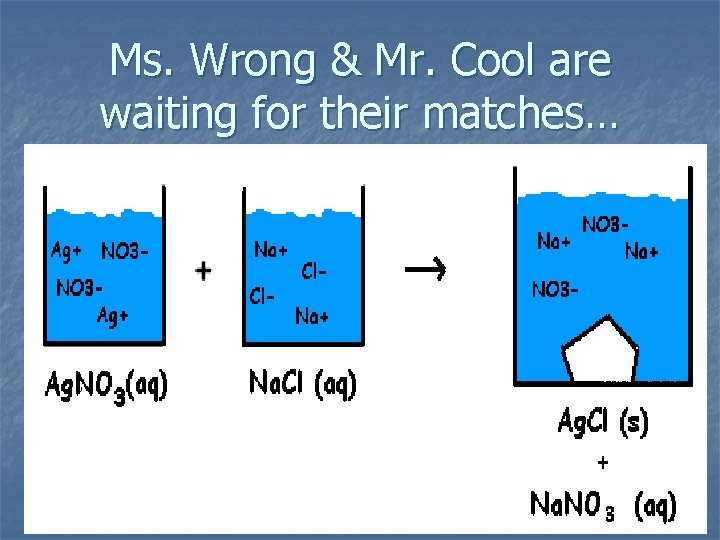
Ms. Wrong & Mr. Cool are waiting for their matches…

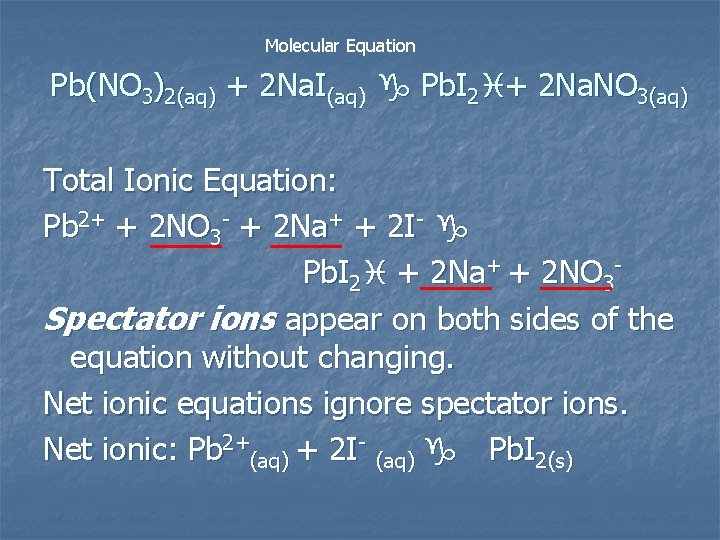
Molecular Equation Pb(NO 3)2(aq) + 2 Na. I(aq) g Pb. I 2 i+ 2 Na. NO 3(aq) Total Ionic Equation: Pb 2+ + 2 NO 3 - + 2 Na+ + 2 I- g Pb. I 2 i + 2 Na+ + 2 NO 3 Spectator ions appear on both sides of the equation without changing. Net ionic equations ignore spectator ions. Net ionic: Pb 2+(aq) + 2 I- (aq) g Pb. I 2(s)

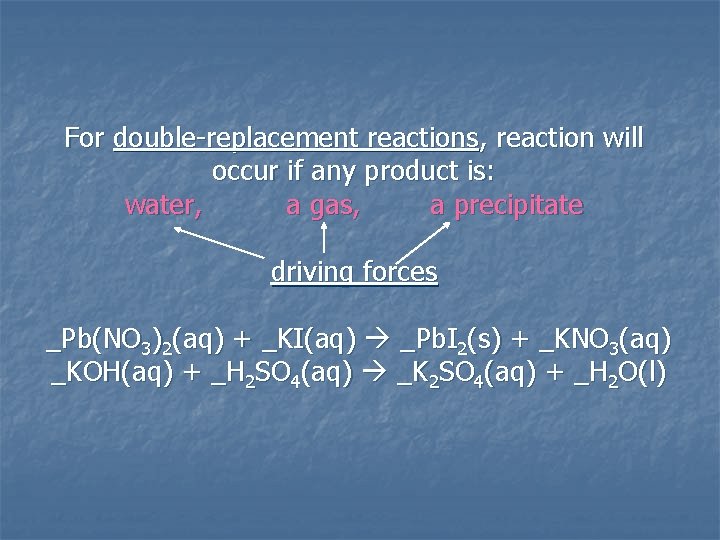
For double-replacement reactions, reaction will occur if any product is: water, a gas, a precipitate driving forces _Pb(NO 3)2(aq) + _KI(aq) _Pb. I 2(s) + _KNO 3(aq) _KOH(aq) + _H 2 SO 4(aq) _K 2 SO 4(aq) + _H 2 O(l)

Try this Ba. Cl 2 ( )+ Na 2 SO 4 ( ) 2 Na. Cl ( ) + Ba. SO 4 ( ) n Total Ionic Equation: n Net Ionic Equation: n Spectator Ions: n Remember: If each product is soluble, no reaction occurs (NR)

Summary on Double Displacement Rxns Remember!!! n Double displacement rxns only happen when 1 of the following is formed: 1. 2. 3. n A precipitate is formed A gas is formed Water is formed In all other cases, the ions stay in solution!!! n No rxn happens!!!