0 D Nanostructures aka Nanoparticles or NPs Review





- Slides: 14

0 -D Nanostructures (aka Nanoparticles or NPs)

Review: What is matter again? Why do we REALLY care about matter? What are some qualities about materials we wish were better?

There are two basic ways you can make nanostructures…what do you suppose they are?

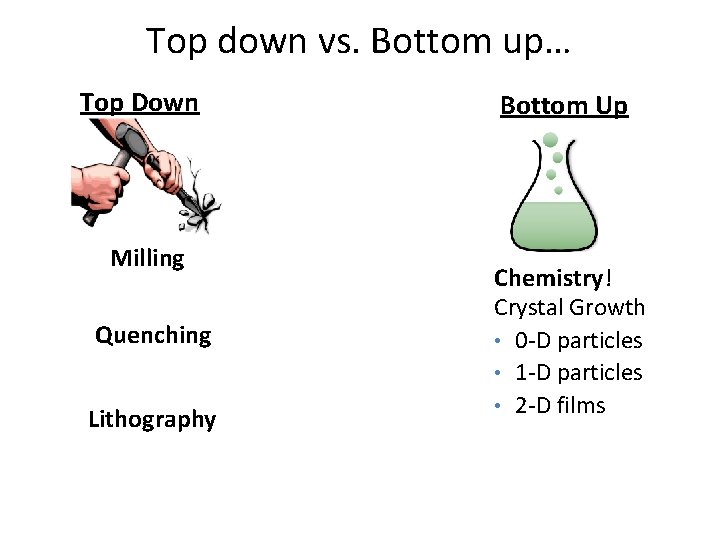
Top down vs. Bottom up… Top Down Milling Quenching Lithography Bottom Up Chemistry! Crystal Growth • 0 -D particles • 1 -D particles • 2 -D films

Top-Down Approaches • Milling (like a rock tumbler) It’s not that great… – Broad size distribution (tens to hundreds of nm) – Varied shape and geometry – Impurities and defects from milling • Repeated quenching (melting, re-solidifying, dissolving, evaporating) It’s not that great either… – Limited to materials with certain specific properties – Difficult to control particle size and shape • Lithography Much better!!

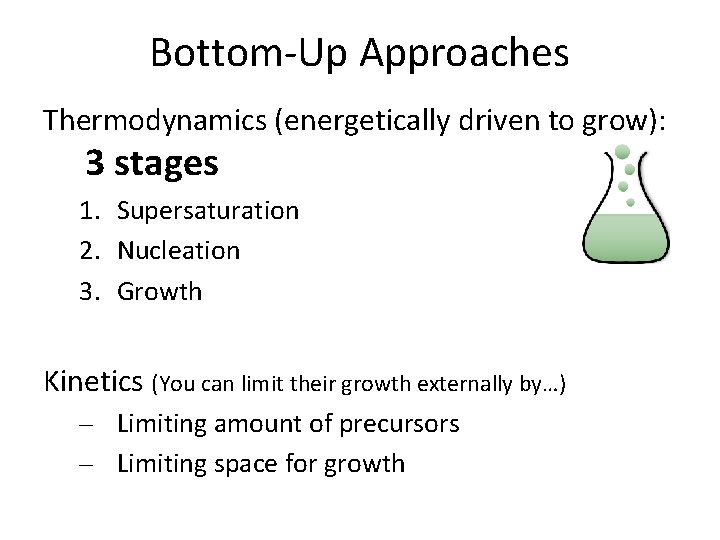
Bottom-Up Approaches Thermodynamics (energetically driven to grow): 3 stages 1. Supersaturation 2. Nucleation 3. Growth Kinetics (You can limit their growth externally by…) – Limiting amount of precursors – Limiting space for growth

You typically WANT… • Uniform size distribution • Uniform morphology • Uniform chemical composition and crystal structure • Monodispersed (NOT AGGLOMERATED!)

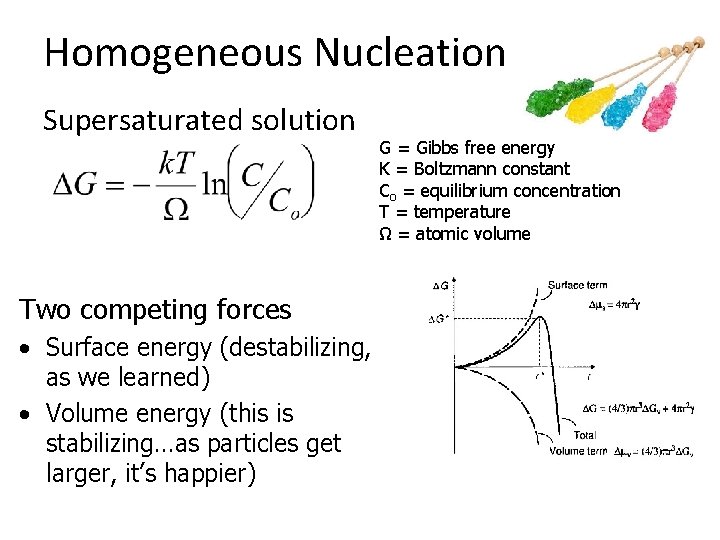
Homogeneous Nucleation Supersaturated solution Two competing forces • Surface energy (destabilizing, as we learned) • Volume energy (this is stabilizing…as particles get larger, it’s happier) G = Gibbs free energy K = Boltzmann constant Co = equilibrium concentration T = temperature Ω = atomic volume

Nucleation Activity: Phenyl Salicylate! Demo: Sodium acetate!

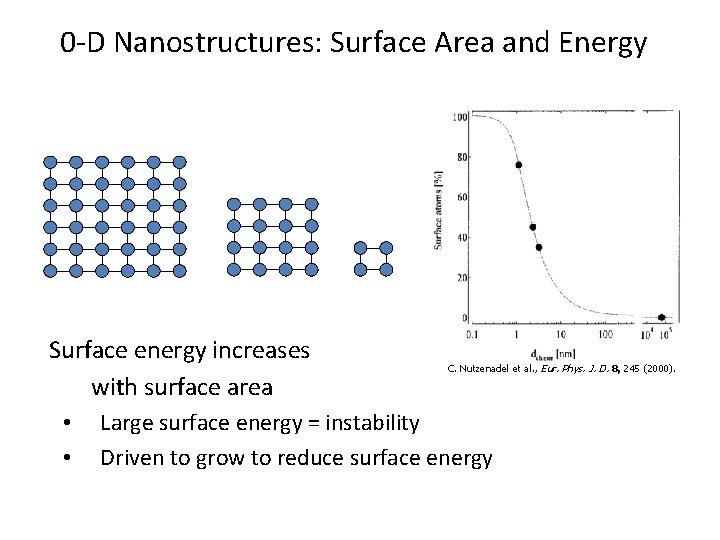
0 -D Nanostructures: Surface Area and Energy Surface energy increases with surface area • • C. Nutzenadel et al. , Eur. Phys. J. D. 8, 245 (2000). Large surface energy = instability Driven to grow to reduce surface energy

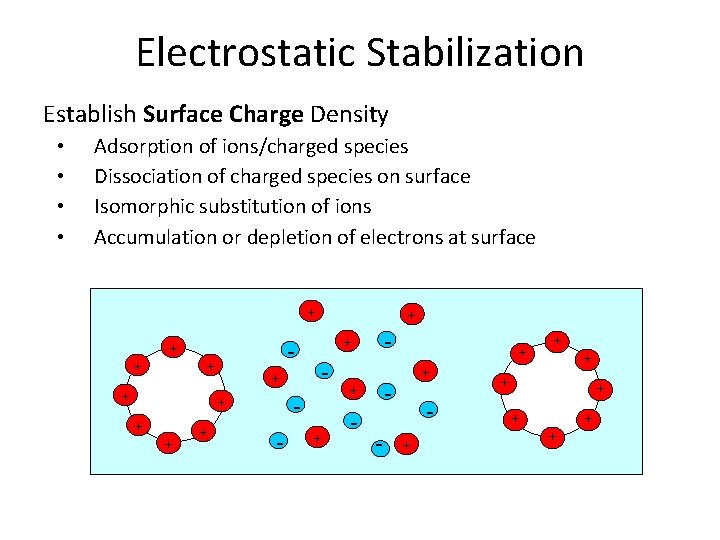
Electrostatic Stabilization Establish Surface Charge Density Adsorption of ions/charged species Dissociation of charged species on surface Isomorphic substitution of ions Accumulation or depletion of electrons at surface + + - - + + + + + + - + - + + + • •

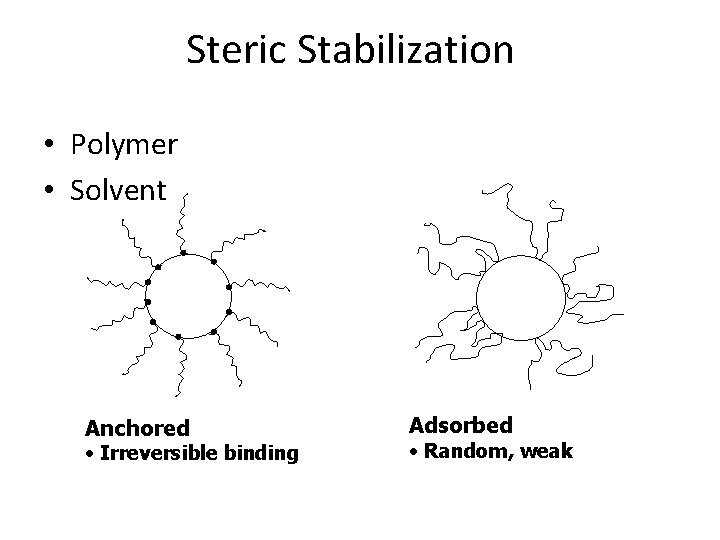
Steric Stabilization • Polymer • Solvent Anchored • Irreversible binding Adsorbed • Random, weak

Metallic NPs

Example: Colloidal Gold • Comprehensive study on synthesis and properties of colloidal gold published by Faraday (1857) • Classic method – Precursor: dilute chlorauric acid – Reducing agent: sodium citrate – Reaction temperature: 100 °C – Product: stable, uniform, ~20 nm particles Other methods use polymeric stabilizers also as a diffusion barrier