WIR SCHAFFEN WISSEN HEUTE FR MORGEN Christophe Delval


- Slides: 15

WIR SCHAFFEN WISSEN – HEUTE FÜR MORGEN • Christophe Delval, Riccardo Iannarelli and Michel J. Rossi Lifetime Extension of Cirrus Cloud Ice Particles upon Contamination with HCl and HNO 3 EGU General Assembly Vienna 2016, Atmospheric Ice Particles in Session AS 3. 3 (Atmospheric Ice Particles), Wednesday, April 20 2016

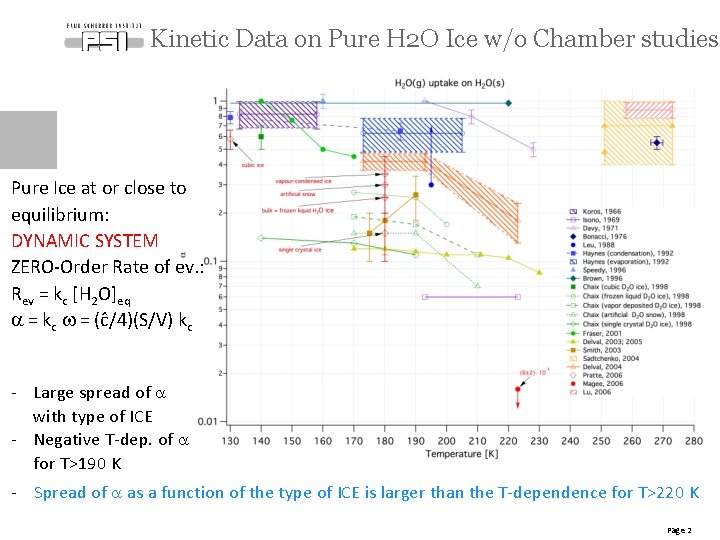
Kinetic Data on Pure H 2 O Ice w/o Chamber studies Pure Ice at or close to equilibrium: DYNAMIC SYSTEM ZERO-Order Rate of ev. : Rev = kc [H 2 O]eq a = kc w = (ĉ/4)(S/V) kc - Large spread of a with type of ICE - Negative T-dep. of a for T>190 K - Spread of a as a function of the type of ICE is larger than the T-dependence for T>220 K Page 2

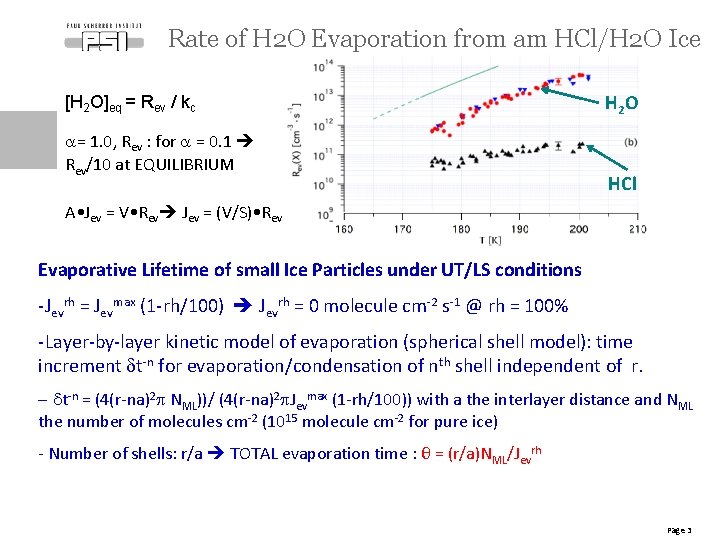
Rate of H 2 O Evaporation from am HCl/H 2 O Ice [H 2 O]eq = Rev / kc a= 1. 0, Rev : for a = 0. 1 Rev/10 at EQUILIBRIUM H 2 O HCl A • Jev = V • Rev Jev = (V/S) • Rev Evaporative Lifetime of small Ice Particles under UT/LS conditions -Jevrh = Jevmax (1 -rh/100) Jevrh = 0 molecule cm-2 s-1 @ rh = 100% -Layer-by-layer kinetic model of evaporation (spherical shell model): time increment dt-n for evaporation/condensation of nth shell independent of r. - dt-n = (4(r-na)2 p NML))/ (4(r-na)2 p. Jevmax (1 -rh/100)) with a the interlayer distance and NML the number of molecules cm-2 (1015 molecule cm-2 for pure ice) - Number of shells: r/a TOTAL evaporation time : θ = (r/a)NML/Jevrh Page 3

Examples: Lifetimes of pure H 2 O Ice Particles • In the kinetic limit the number of shells (molecular layers) evaporating or condensing per s is constant and independent of r • Kinetic limit @ a = 1. 0 is in the range 0. 3 and 1 mm, with a = 0. 1 it is 3 -10 mm • Input Data: r = 10 mm (spherical particle), a = 0. 25 nm (4 bilayers of ice per nm or 4 • 104 layers for 10 mm radius), rh = 80%, Jevmax = 5 • 1016 molecule cm-2 s-1 (pure ice, 200 K), (S/V) = 1/1028, NLM = 1015 molecule cm-2. • Total time to evaporation of particle: θ = (1015 • 4 • 104)/(5 • 1014 • 1028 • 0. 2) leads to θ = 4000 s or 67 minutes at 80% rh. • For 95% rh one obtains θ = 4 • 4000 = 16’ 000 s or 267 minutes or 4. 4 hours, @ ice saturation θ is infinity, at 0%rh θ = 800 s or 13. 3 minutes. For a = 1. 0 θ = 80 s @ 0% rh (Too short for any heterogeneous chemistry to take place). • Conclusion: Large span of total evaporation time θ of a pure ice particle ranging from one minute to 5 hours depending on a and rh! Page 4

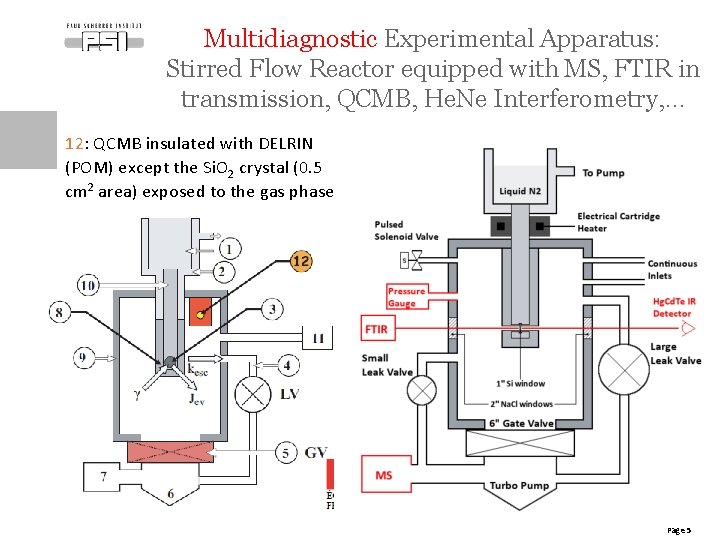
Multidiagnostic Experimental Apparatus: Stirred Flow Reactor equipped with MS, FTIR in transmission, QCMB, He. Ne Interferometry, … 12: QCMB insulated with DELRIN (POM) except the Si. O 2 crystal (0. 5 cm 2 area) exposed to the gas phase Page 5

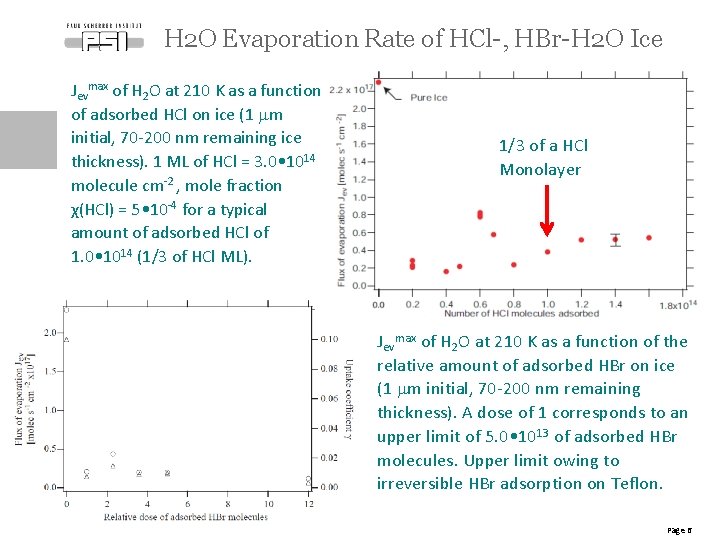
H 2 O Evaporation Rate of HCl-, HBr-H 2 O Ice Jevmax of H 2 O at 210 K as a function of adsorbed HCl on ice (1 mm initial, 70 -200 nm remaining ice thickness). 1 ML of HCl = 3. 0 • 1014 molecule cm-2 , mole fraction χ(HCl) = 5 • 10 -4 for a typical amount of adsorbed HCl of 1. 0 • 1014 (1/3 of HCl ML). 1/3 of a HCl Monolayer Jevmax of H 2 O at 210 K as a function of the relative amount of adsorbed HBr on ice (1 mm initial, 70 -200 nm remaining thickness). A dose of 1 corresponds to an upper limit of 5. 0 • 1013 of adsorbed HBr molecules. Upper limit owing to irreversible HBr adsorption on Teflon. Page 6

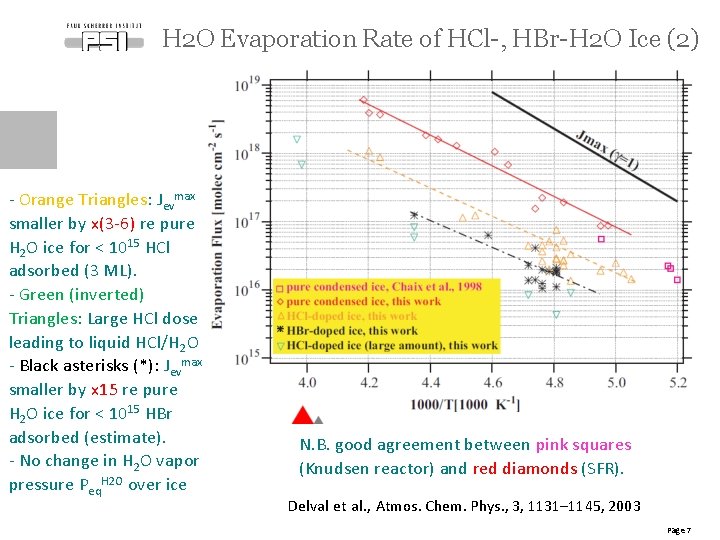
H 2 O Evaporation Rate of HCl-, HBr-H 2 O Ice (2) - Orange Triangles: Jevmax smaller by x(3 -6) re pure H 2 O ice for < 1015 HCl adsorbed (3 ML). - Green (inverted) Triangles: Large HCl dose leading to liquid HCl/H 2 O - Black asterisks (*): Jevmax smaller by x 15 re pure H 2 O ice for < 1015 HBr adsorbed (estimate). - No change in H 2 O vapor pressure Peq. H 2 O over ice N. B. good agreement between pink squares (Knudsen reactor) and red diamonds (SFR). Delval et al. , Atmos. Chem. Phys. , 3, 1131– 1145, 2003 Page 7

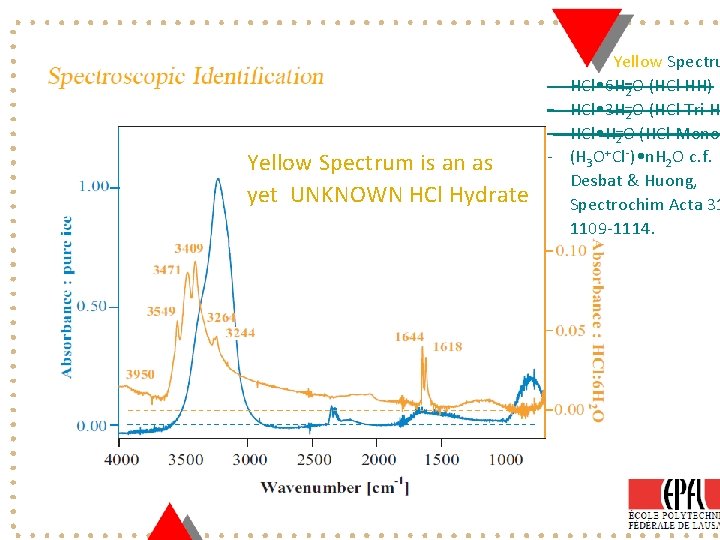
Yellow Spectrum is an as yet UNKNOWN HCl Hydrate - Yellow Spectru HCl • 6 H 2 O (HCl HH) HCl • 3 H 2 O (HCl Tri H HCl • H 2 O (HCl Mono (H 3 O+Cl-) • n. H 2 O c. f. Desbat & Huong, Spectrochim Acta 31 1109 -1114. Page 8

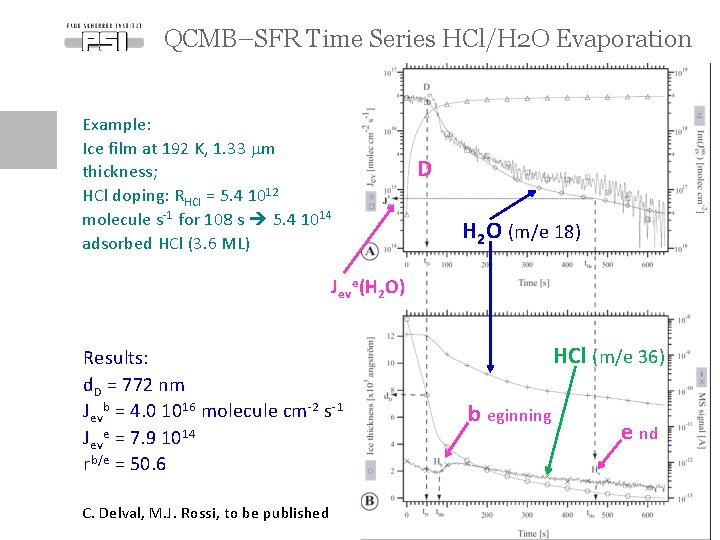
QCMB–SFR Time Series HCl/H 2 O Evaporation Example: Ice film at 192 K, 1. 33 mm thickness; HCl doping: RHCl = 5. 4 1012 molecule s-1 for 108 s 5. 4 1014 adsorbed HCl (3. 6 ML) D H 2 O (m/e 18) Jeve(H 2 O) Results: d. D = 772 nm Jevb = 4. 0 1016 molecule cm-2 s-1 Jeve = 7. 9 1014 rb/e = 50. 6 HCl (m/e 36) b eginning e nd C. Delval, M. J. Rossi, to be published Page 9

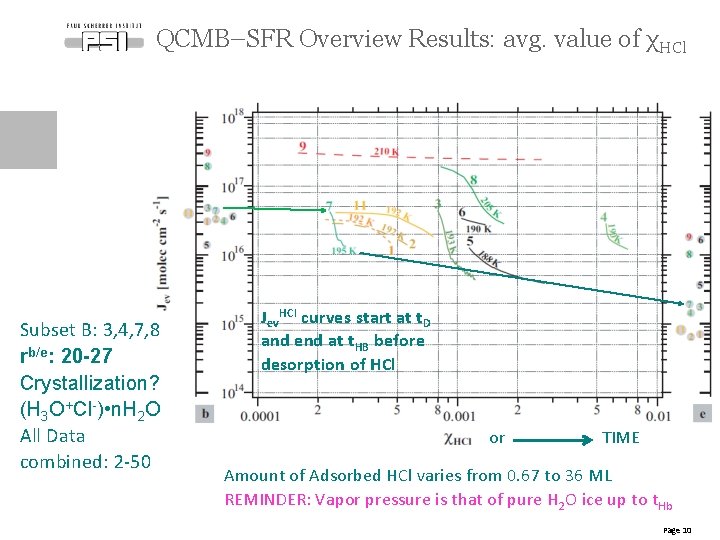
QCMB–SFR Overview Results: avg. value of χHCl Subset B: 3, 4, 7, 8 rb/e: 20 -27 Crystallization? (H 3 O+Cl-) • n. H 2 O All Data combined: 2 -50 Jev. HCl curves start at t. D and end at t. HB before desorption of HCl or TIME Amount of Adsorbed HCl varies from 0. 67 to 36 ML REMINDER: Vapor pressure is that of pure H 2 O ice up to t. Hb Page 10

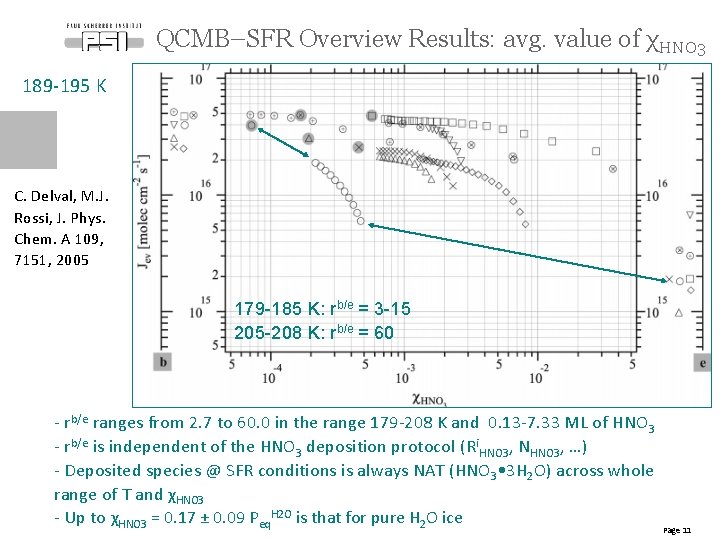
QCMB–SFR Overview Results: avg. value of χHNO 3 189 -195 K C. Delval, M. J. Rossi, J. Phys. Chem. A 109, 7151, 2005 179 -185 K: rb/e = 3 -15 205 -208 K: rb/e = 60 - rb/e ranges from 2. 7 to 60. 0 in the range 179 -208 K and 0. 13 -7. 33 ML of HNO 3 - rb/e is independent of the HNO 3 deposition protocol (Ri. HNO 3, NHNO 3, …) - Deposited species @ SFR conditions is always NAT (HNO 3 • 3 H 2 O) across whole range of T and χHNO 3 - Up to χHNO 3 = 0. 17 ± 0. 09 Peq. H 2 O is that for pure H 2 O ice Page 11

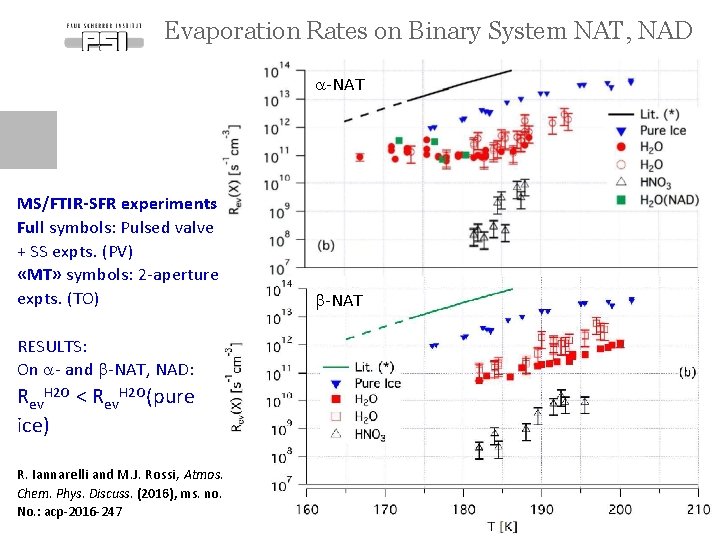
Evaporation Rates on Binary System NAT, NAD a-NAT MS/FTIR-SFR experiments Full symbols: Pulsed valve + SS expts. (PV) «MT» symbols: 2 -aperture expts. (TO) b-NAT RESULTS: On a- and b-NAT, NAD: Rev. H 2 O < Rev. H 2 O(pure ice) R. Iannarelli and M. J. Rossi, Atmos. Chem. Phys. Discuss. (2016), ms. no. No. : acp-2016 -247 Page 12

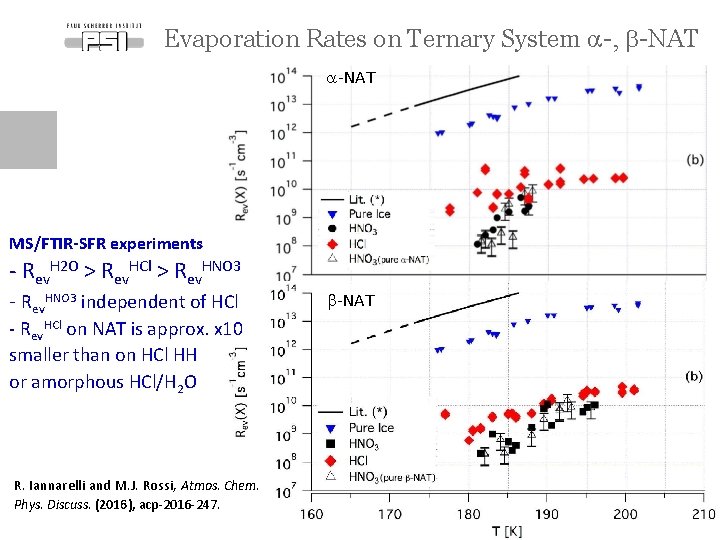
Evaporation Rates on Ternary System a-, b-NAT a-NAT MS/FTIR-SFR experiments - Rev. H 2 O > Rev. HCl > Rev. HNO 3 - Rev. HNO 3 independent of HCl b-NAT - Rev. HCl on NAT is approx. x 10 smaller than on HCl HH or amorphous HCl/H 2 O R. Iannarelli and M. J. Rossi, Atmos. Chem. Phys. Discuss. (2016), acp-2016 -247. Page 13

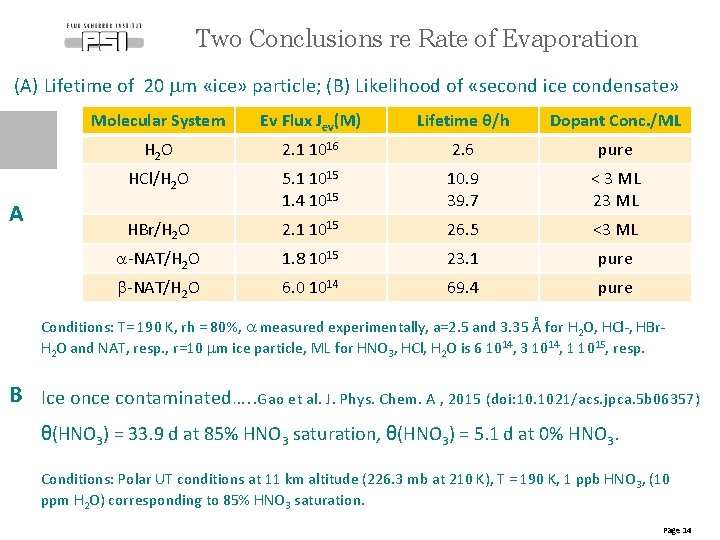
Two Conclusions re Rate of Evaporation (A) Lifetime of 20 mm «ice» particle; (B) Likelihood of «second ice condensate» A Molecular System Ev Flux Jev(M) Lifetime θ/h Dopant Conc. /ML H 2 O 2. 1 1016 2. 6 pure HCl/H 2 O 5. 1 1015 1. 4 1015 10. 9 39. 7 < 3 ML 23 ML HBr/H 2 O 2. 1 1015 26. 5 <3 ML a-NAT/H 2 O 1. 8 1015 23. 1 pure b-NAT/H 2 O 6. 0 1014 69. 4 pure Conditions: T= 190 K, rh = 80%, a measured experimentally, a=2. 5 and 3. 35 Å for H 2 O, HCl-, HBr. H 2 O and NAT, resp. , r=10 mm ice particle, ML for HNO 3, HCl, H 2 O is 6 1014, 3 1014, 1 1015, resp. B Ice once contaminated…. . Gao et al. J. Phys. Chem. A , 2015 (doi: 10. 1021/acs. jpca. 5 b 06357) θ(HNO 3) = 33. 9 d at 85% HNO 3 saturation, θ(HNO 3) = 5. 1 d at 0% HNO 3. Conditions: Polar UT conditions at 11 km altitude (226. 3 mb at 210 K), T = 190 K, 1 ppb HNO 3, (10 ppm H 2 O) corresponding to 85% HNO 3 saturation. Page 14

Wir schaffen Wissen – heute für morgen Relative Humidity and accommodation/evaporation dynamics control ice cloud lifetimes in UT/LS. Once contaminated ice remains «stained» with atmospheric contaminants during its existence in the atmosphere. Page 15
 Die merkelraute
Die merkelraute Zylinderprinzip
Zylinderprinzip Guten morgen ein neuer tag
Guten morgen ein neuer tag Leuwiko
Leuwiko Was du heute machen kannst verschiebe nicht auf morgen
Was du heute machen kannst verschiebe nicht auf morgen Dachber
Dachber Winter kommt winter kommt flocken fallen nieder lied
Winter kommt winter kommt flocken fallen nieder lied Bths prefect
Bths prefect Was haben wir heute
Was haben wir heute Wievielten haben wir heute
Wievielten haben wir heute Zünden wir ein lichtlein an
Zünden wir ein lichtlein an Wir sind nicht mehr was wir mal waren
Wir sind nicht mehr was wir mal waren Zünden wir ein lichtlein an
Zünden wir ein lichtlein an Wissen aneignen
Wissen aneignen Ergänzen sie die präpositionen
Ergänzen sie die präpositionen Es ist nicht genug zu wissen
Es ist nicht genug zu wissen





























