What is Radioactivity Radioactivity is The disintegration of








- Slides: 10

What is Radioactivity?

Radioactivity is. . . The disintegration of the nucleus of an isotope (form of an element) is called radioactivity. Isotopes with unstable nuclei, or radioisotopes, degrade or disintegrate into different isotopes with release of subatomic particles or energy. Ionizing radiation is high energy radiation and can be damaging.

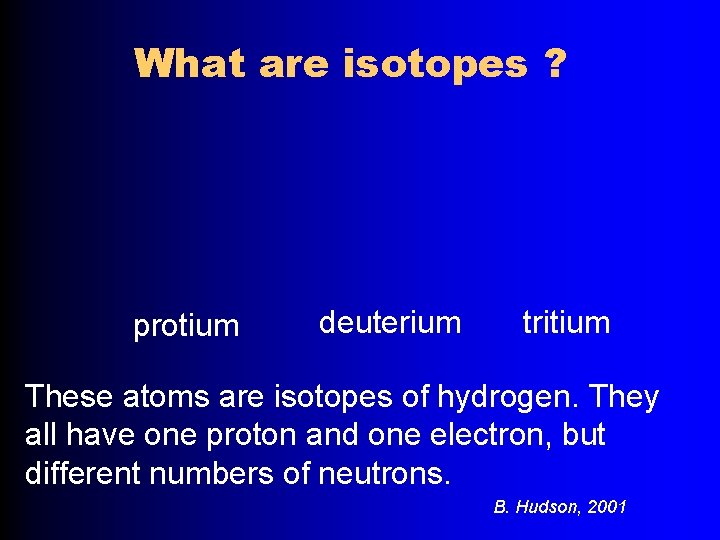
What are isotopes ? protium deuterium tritium These atoms are isotopes of hydrogen. They all have one proton and one electron, but different numbers of neutrons. B. Hudson, 2001

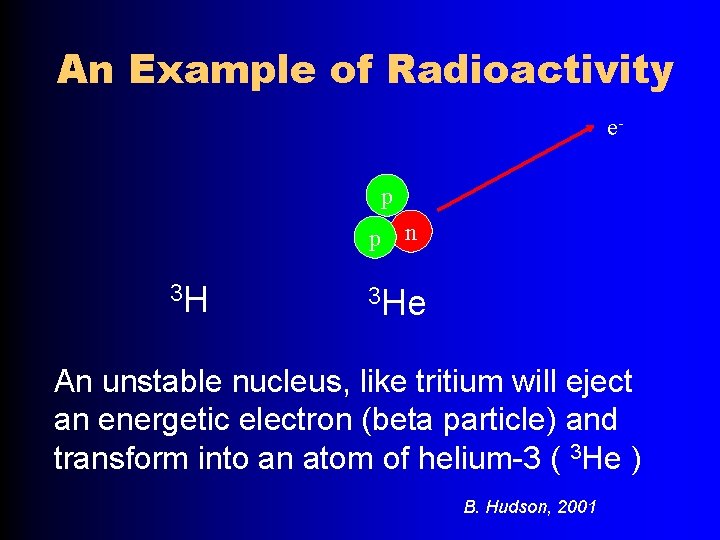
An Example of Radioactivity ep p 3 H n 3 He An unstable nucleus, like tritium will eject an energetic electron (beta particle) and transform into an atom of helium-3 ( 3 He ) B. Hudson, 2001

What are common types of ionizing radiation? Alpha Fast moving Positively charged 4 He+2 Beta High energy negative charge High speed electron emission Gamma High energy Electromagnetic radiation

What is a half-life? The time it takes for an unstable isotope to degrade to another isotope. This usually means that you have a completely different element!

GO TO STUDENT BEAN ACTIVITY AND COME BACK TO LAST SLIDES LATER

So. . . does this mean that radioactivity is forever? If a rabbit jumps halfway to his hole each time will he ever get in? Actually, at some point our fuzzy friend will just fall in the hole no matter what you hypothesize!

The same is true for radioactive elements. . . After 10 half-lives, an element is considered to be completely degraded!

So. . . . How long will carbon-14 with a halflife of around 5000 years be hanging around? (how old can something be for carbon dating to work? )
 Radioactivity as spontaneous disintegration
Radioactivity as spontaneous disintegration Usp weight variation limits
Usp weight variation limits Theory of positive disintegration
Theory of positive disintegration Disintegrating tablet meaning
Disintegrating tablet meaning Dissolution vs disintegration
Dissolution vs disintegration Tablets quality control tests
Tablets quality control tests Temporal pole function
Temporal pole function Theory of positive disintegration
Theory of positive disintegration Natural radioactivity
Natural radioactivity Who discovered radioactivity
Who discovered radioactivity Key terms radioactivity and nuclear reactions
Key terms radioactivity and nuclear reactions


















