Water Water H 2 O or HOH is




- Slides: 6

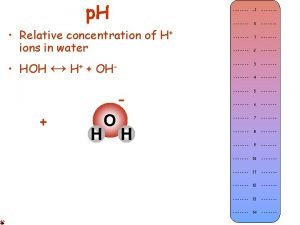
Water

Water ( H 2 O or HOH ) is the most abundant molecule on Earth – good thing. . . it is essential for all life. It is also the only substance found naturally on our planet in all 3 common phases of matter – solid, liquid and gas. Water is called the "universal solvent" because it dissolves more substances than any other liquid. This means that wherever water goes, either through the ground or through our bodies, it takes along valuable chemicals, minerals, and nutrients. Solution – A type of mixture formed when one substance is dissolved into another resulting in an even distribution. Solvent – A liquid (or gas) that dissolves other smaller amounts of liquids, solids and gasses into it. Solute – The substance in a solution (smaller amount) which is dissolved into the solvent. Concentration – The ratio of a solute compared to the solvent in a mixture. Solubility – The ability of a substance to be dissolved into a solution. Saturation – The point at which the maximum amount of a solute can be dissolved while still maintaining a solution with even distribution. ? ? ? ? What is the solvent in the solution we call the Earth’s Atmosphere? Mr. Lovett Rocks

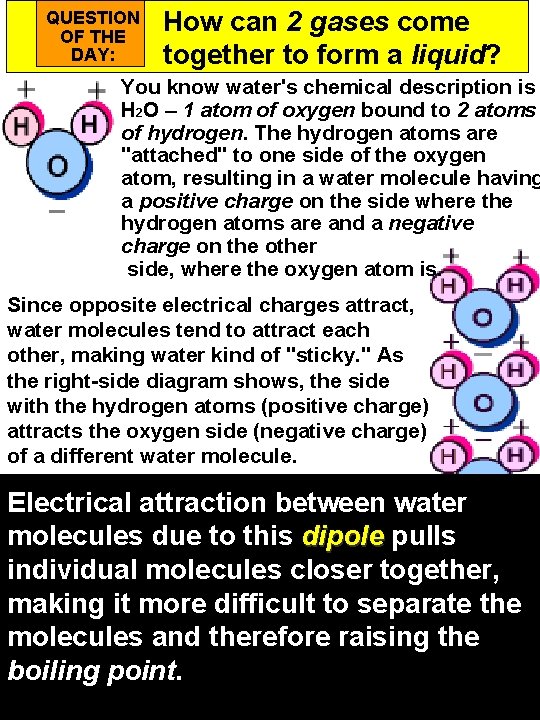
QUESTION OF THE DAY: How can 2 gases come together to form a liquid? You know water's chemical description is H 2 O – 1 atom of oxygen bound to 2 atoms of hydrogen. The hydrogen atoms are "attached" to one side of the oxygen atom, resulting in a water molecule having a positive charge on the side where the hydrogen atoms are and a negative charge on the other side, where the oxygen atom is. Since opposite electrical charges attract, water molecules tend to attract each other, making water kind of "sticky. " As the right-side diagram shows, the side with the hydrogen atoms (positive charge) attracts the oxygen side (negative charge) of a different water molecule. Electrical attraction between water molecules due to this dipole pulls individual molecules closer together, making it more difficult to separate the molecules and therefore raising the boiling point.

Capillary Action - The movement of water within spaces due to the forces of adhesion (H 2 O molecules stick to other substances), cohesion (H 2 O molecules stick together), and surface tension. Plants and trees couldn't thrive without capillary action. Water, which contains dissolved nutrients, gets inside the roots and starts climbing up the plant tissue. As water molecule #1 starts climbing, it pulls along water molecule #2, which, of course, is dragging water molecule #3, and so on. Surface Tension -A measure of the strength of the water's surface film because of the attraction between the water molecules. This surface tension permits water to hold up substances heavier and denser than itself. Some aquatic insects such as the water strider rely on surface tension to walk on water.

FACTS: -Because ice floats, we can infer that ice must be less dense than water. -If water is frozen in a glass jar, the glass jar breaks. Conclusion: The volume of ice must be greater than the same mass of liquid water. Why does the volume increase? Usually, as temperature decreases, density increases ( the molecules become more closely packed) This pattern does not hold true for ice -the exact opposite occurs! Notice the empty spaces within the ice structure, as this translates to a more open or expanded structure. The ice structure takes up more volume than the liquid water molecules, hence is less dense than liquid water.

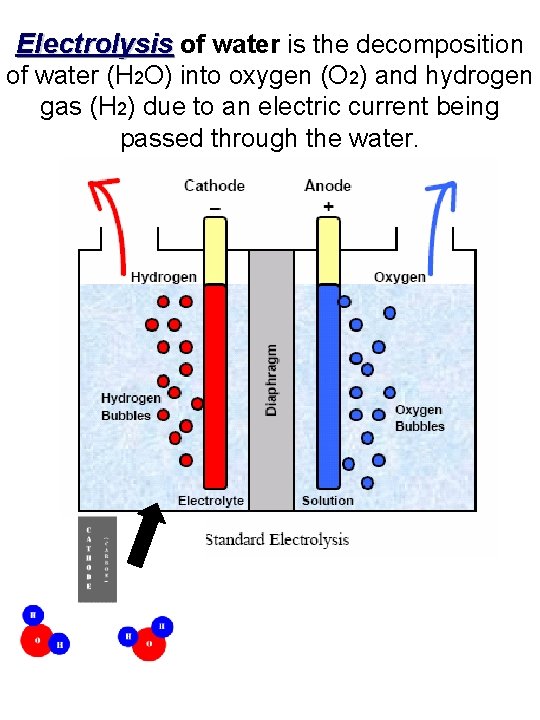
Electrolysis of water is the decomposition of water (H 2 O) into oxygen (O 2) and hydrogen gas (H 2) due to an electric current being passed through the water.