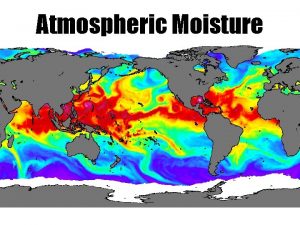
Water in the Atmosphere Section 1 Atmospheric Moisture


















- Slides: 22

Water in the Atmosphere Section 1: Atmospheric Moisture Preview • Key Ideas • Changing Forms of Water • Humidity • Measuring Relative Humidity Section 1

Water in the Atmosphere Section 1 Key Ideas • Explain how heat energy affects the changing phases of water. • Explain what absolute humidity and relative humidity are, and describe how they are measured. • Describe what happens when the temperature of air decreases to the dew point or below.

Water in the Atmosphere Section 1 Changing Forms of Water • Water in the atmosphere exists in three states, or phases. • One phase is known as a gas called water vapor. • The other two phases of water are the solid phase known as ice and the liquid phase known as water. • Water changes from one phase to another when heat energy is absorbed or released.

Water in the Atmosphere Section 1 Changing Forms of Water, continued Latent Heat • latent heat the heat energy that is absorbed or released by a substance during a phase change • When liquid water evaporates, the water absorbs energy from the environment. • When water vapor changes back into a liquid through the process of condensation, energy is released into the surrounding air and the molecules move closer together. • Latent heat is absorbed when ice thaws, and latent heat is released when water freezes.

Water in the Atmosphere Section 1 Changing Forms of Water, continued Evaporation • Most water enters the atmosphere through evaporation of ocean water near the equator. • Water vapor also enters the atmosphere by evaporation from lakes, ponds, streams, and soil. • Plants release water into the atmosphere in a process called transpiration. • Volcanoes and burning fuels also release small amounts of water vapor into the atmosphere.

Water in the Atmosphere Section 1 Changing Forms of Water, continued Sublimation • sublimation the process in which a solid changes directly into a gas (the term is sometimes also used for the reverse process) • Ice commonly changes into a liquid before changing into a gas. • When the air is dry and the temperature is below freezing, ice and snow may sublimate into water vapor. • Water vapor can also turn directly into ice without becoming a liquid.

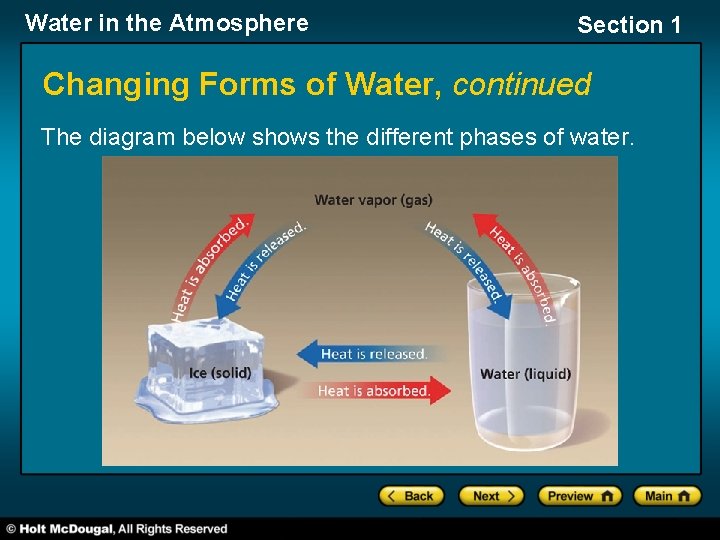
Water in the Atmosphere Section 1 Changing Forms of Water, continued The diagram below shows the different phases of water.

Water in the Atmosphere Section 1 Reading Check Summarize the conditions under which sublimation commonly occurs. When air is very dry and the temperature is below freezing, ice and snow change directly into water vapor by sublimation.

Water in the Atmosphere Section 1 Humidity • Water vapor in the atmosphere is known as humidity. • Humidity is controlled by rates of condensation and evaporation. • The rate of evaporation is determined by the temperature of the air. The higher the temperature is, the higher the rate of evaporation is. • The rate of condensation is determined by vapor pressure. When vapor pressure is high, the condensation rate is high.

Water in the Atmosphere Section 1 Humidity, continued • When the rate of evaporation and the rate of condensation are in equilibrium, the air is said to be “saturated. ” • dew point at constant pressure and water vapor content, the temperature at which the rate of condensation equals the rate of evaporation • At temperatures below the dew point, net condensation occurs, and liquid water droplets form.

Water in the Atmosphere Section 1 Humidity, continued Absolute Humidity absolute humidity the mass of water vapor per unit volume of air that contains the water vapor, usually expressed as grams of water vapor per cubic meter of air

Water in the Atmosphere Section 1 Humidity, continued Absolute Humidity, continued • However, as air moves, its volume changes as a result of temperature and pressure changes. • Therefore, meteorologists prefer to describe humidity by using the mixing ratio of air. • The mixing ratio of air is the mass of water vapor in a unit of air relative to the mass of the dry air. • Because this measurement uses only units of mass, it is not affected by changes in temperature or pressure.

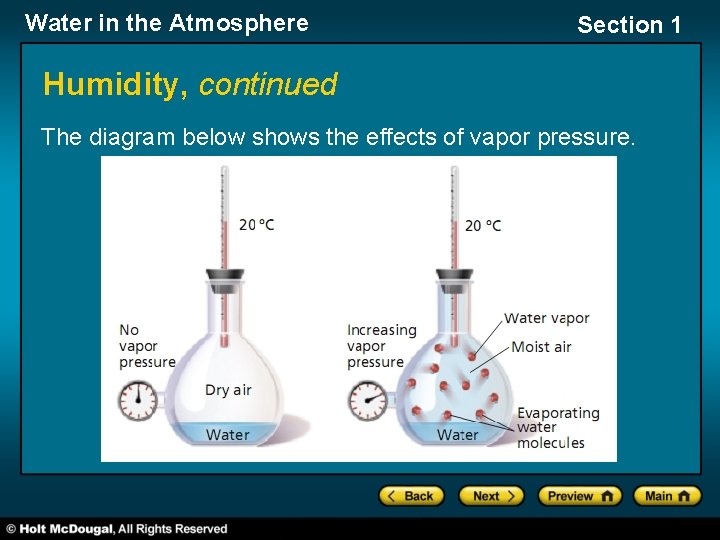
Water in the Atmosphere Section 1 Humidity, continued The diagram below shows the effects of vapor pressure.

Water in the Atmosphere Section 1 Humidity, continued Relative Humidity • relative humidity the ratio of the amount of water vapor in the air to the amount of water vapor needed to reach saturation at a given temperature • If the temperature does not change, the relative humidity will increase if moisture enters the air. • Relative humidity can also increase if the moisture in the air remains constant but the temperature decreases. • If the temperature increases as the moisture in the air remains constant, the relative humidity will decrease.

Water in the Atmosphere Section 1 Humidity, continued Reaching the Dew Point • When the air is nearly saturated with a relative humidity of almost 100%, only a small temperature drop is needed for air to reach its dew point. • Air may cool to its dew point by conduction when the air comes in contact with a cold surface. • During the night, grass, leaves, and other objects near the ground lose heat. When the temperature of air cools below the dew point, condensation called dew is formed.

Water in the Atmosphere Section 1 Humidity, continued Reaching the Dew Point, continued • If dew point falls below the freezing temperature of water, water vapor may change directly into solid ice crystals, or frost. • Because frost forms when water vapor turns directly into ice, frost is not frozen dew. • Frozen dew is relatively uncommon. Unlike frost, frozen dew forms as clear beads of ice.

Water in the Atmosphere Section 1 Reading Check How does dew differ from frost? Dew is liquid moisture that condenses from air on cool objects when the air is nearly saturated and the temperature drops. Frost is water vapor that condenses as ice crystals onto a cool surface directly from the air when the dew point is below freezing.

Water in the Atmosphere Section 1 Measuring Humidity Using an Electrical Hygrometer • Humidity is commonly measured by a humidity sensor that uses a thin polymer film. • The polymer film’s ability to conduct electricity is affected by the relative humidity of the surrounding air. • Thus, by measuring the polymer film’s ability to store electricity, relative humidity can be determined.

Water in the Atmosphere Section 1 Measuring Humidity, continued Using a Psychrometer • A psychrometer consists of two identical thermometers. The bulb of one thermometer is covered with a damp wick. The bulb of the othermometer remains dry. • When the psychrometer is held by a handle and whirled through the air, the air circulates around both thermometers. • The difference between the dry-bulb temperature and the wet-bulb temperature is used to calculate relative humidity.

Water in the Atmosphere Section 1 Measuring Humidity, continued Other Methods for Measuring Humidity • A dew cell consist of a ceramic cylinder with electrodes attached to it and treated with lithium chloride, Li. Cl. • By detecting the electrical resistance of Li. Cl as it is heated and cooled, the dew cell can determine dew point. • The hair hygrometer determines relative humidity based on the principle that hair becomes longer as relative humidity increases.

Water in the Atmosphere Section 1 Measuring Humidity, continued Measuring Humidity at High Altitudes • The hygrometer may be carried up into the atmosphere in an instrument package known as a radiosonde. • The electric hygrometer is triggered by passing electric current through a moisture-attracting chemical substance. • The amount of moisture changes the electrical conductivity of the chemical substance. The change can then be expressed as the relative humidity of the surrounding air.

Water in the Atmosphere Measuring Relative Humidity Click below to watch the Visual Concept. Section 1
 Lab 5 atmospheric moisture
Lab 5 atmospheric moisture Water and water and water water
Water and water and water water The four main layers of the atmosphere
The four main layers of the atmosphere Convective rain
Convective rain Water vapor in the atmosphere percentage
Water vapor in the atmosphere percentage Atmosphere
Atmosphere Atmospheric opacity
Atmospheric opacity Atmospheric perspective watercolor
Atmospheric perspective watercolor Atmospheric suits
Atmospheric suits Atmospheric gravity waves
Atmospheric gravity waves Penn state department of meteorology
Penn state department of meteorology Pressure at different altitudes
Pressure at different altitudes Forceparcel
Forceparcel Single cell model of atmospheric circulation
Single cell model of atmospheric circulation Atmospheric stability
Atmospheric stability Mechanical equilibrium definition
Mechanical equilibrium definition Conditionally unstable atmosphere
Conditionally unstable atmosphere Single cell model of atmospheric circulation
Single cell model of atmospheric circulation Atmospheric heaven
Atmospheric heaven Atmospheric opacity
Atmospheric opacity Atmospheric circulation
Atmospheric circulation Atmospheric distortion correction
Atmospheric distortion correction What is weather variables
What is weather variables