VSEPR 10 1 Valence shell electron pair repulsion
- Slides: 18

VSEPR

10. 1

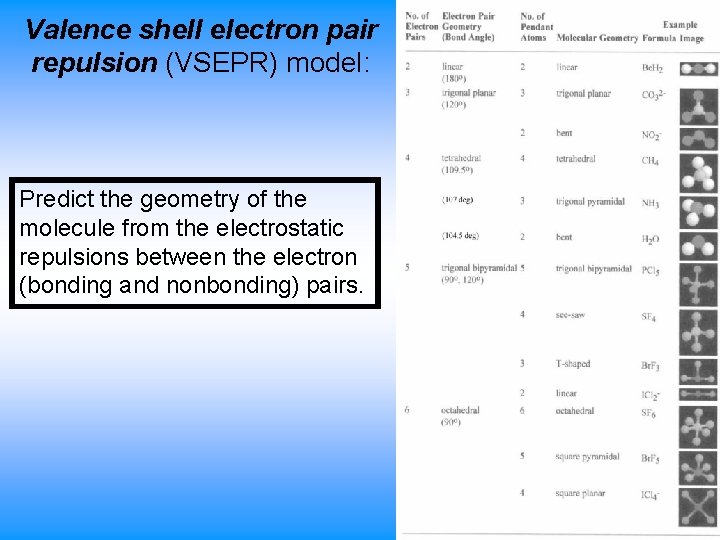
Valence shell electron pair repulsion (VSEPR) model: Predict the geometry of the molecule from the electrostatic repulsions between the electron (bonding and nonbonding) pairs.

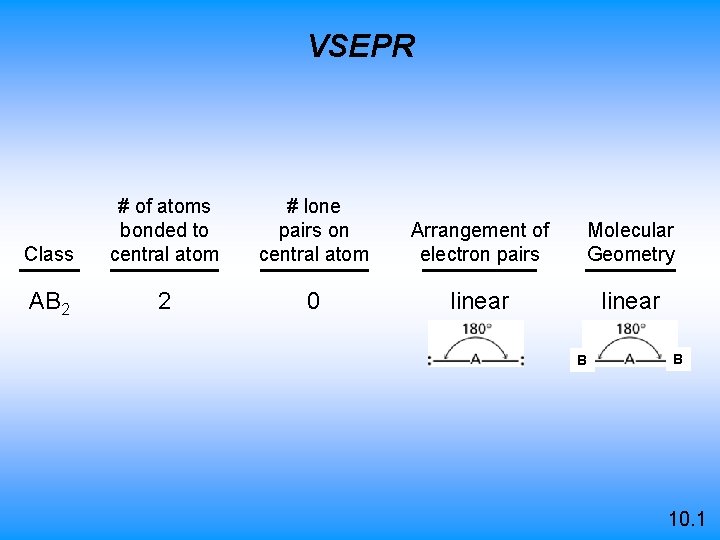
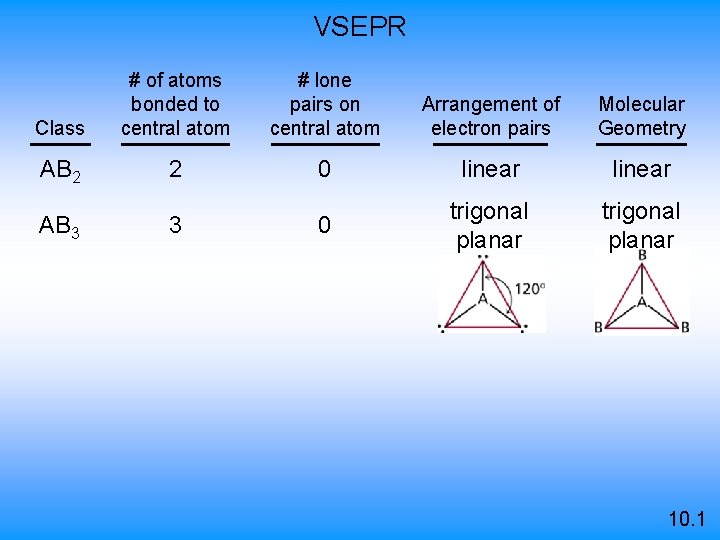
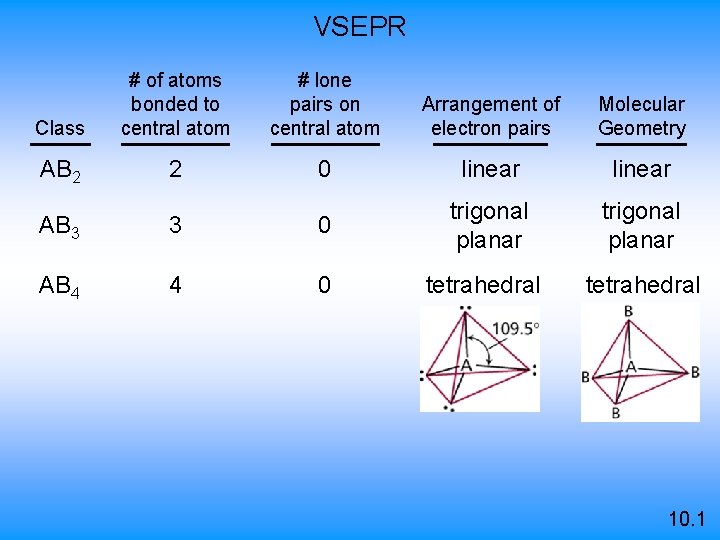
VSEPR Class # of atoms bonded to central atom # lone pairs on central atom Arrangement of electron pairs Molecular Geometry AB 2 2 0 linear B B 10. 1

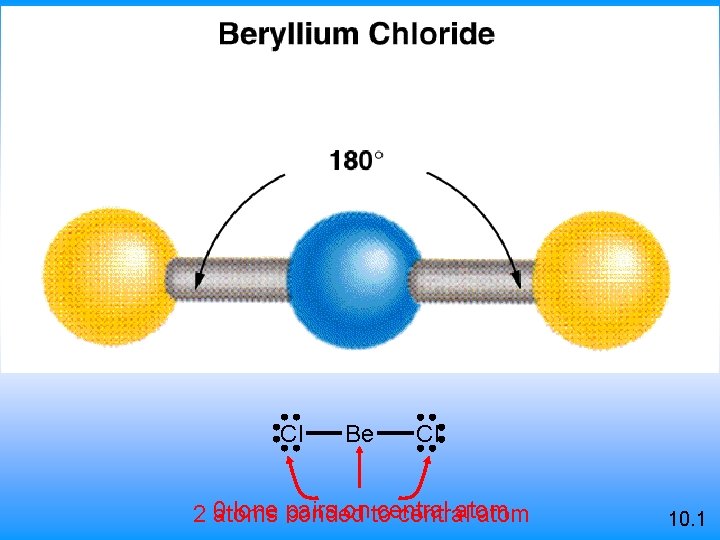
Cl Be Cl 0 lone bonded pairs on to central atom 2 atoms central atom 10. 1

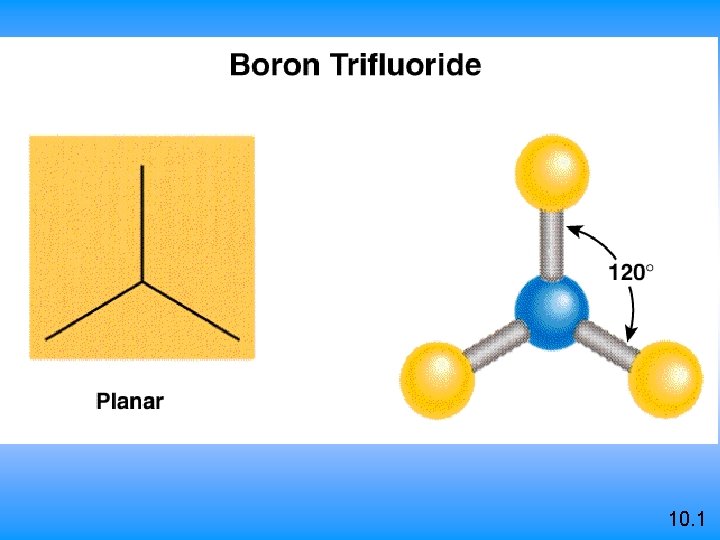
VSEPR Class # of atoms bonded to central atom # lone pairs on central atom Arrangement of electron pairs Molecular Geometry AB 2 2 0 linear 0 trigonal planar AB 3 3 10. 1

10. 1

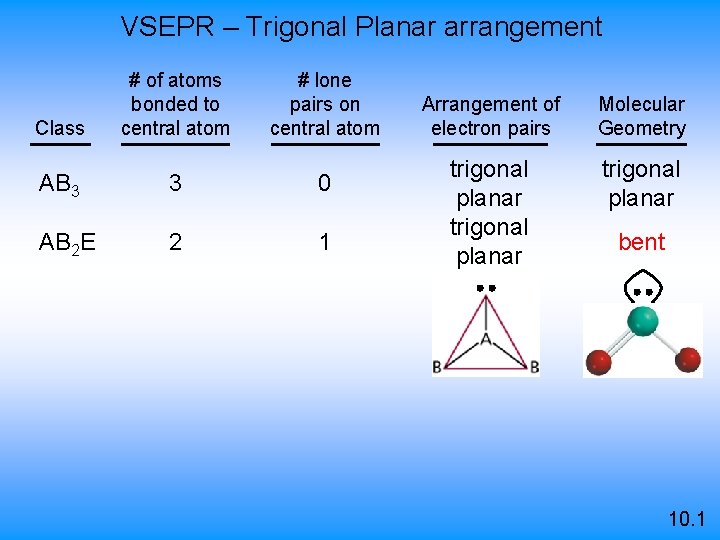
VSEPR – Trigonal Planar arrangement Class # of atoms bonded to central atom # lone pairs on central atom AB 3 3 0 AB 2 E 2 1 Arrangement of electron pairs Molecular Geometry trigonal planar bent 10. 1

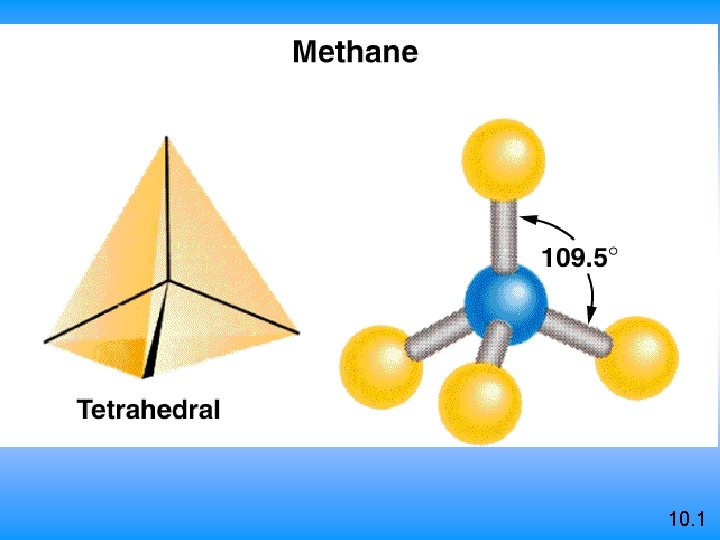
VSEPR Class # of atoms bonded to central atom # lone pairs on central atom Arrangement of electron pairs Molecular Geometry AB 2 2 0 linear trigonal planar tetrahedral AB 3 3 0 trigonal planar AB 4 4 0 tetrahedral 10. 1

10. 1

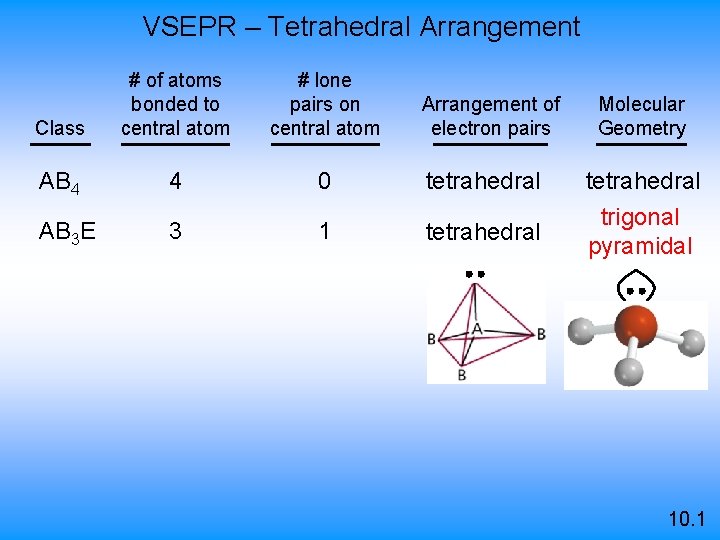
VSEPR – Tetrahedral Arrangement Class # of atoms bonded to central atom # lone pairs on central atom AB 4 4 0 AB 3 E 3 1 Arrangement of electron pairs Molecular Geometry tetrahedral trigonal pyramidal 10. 1

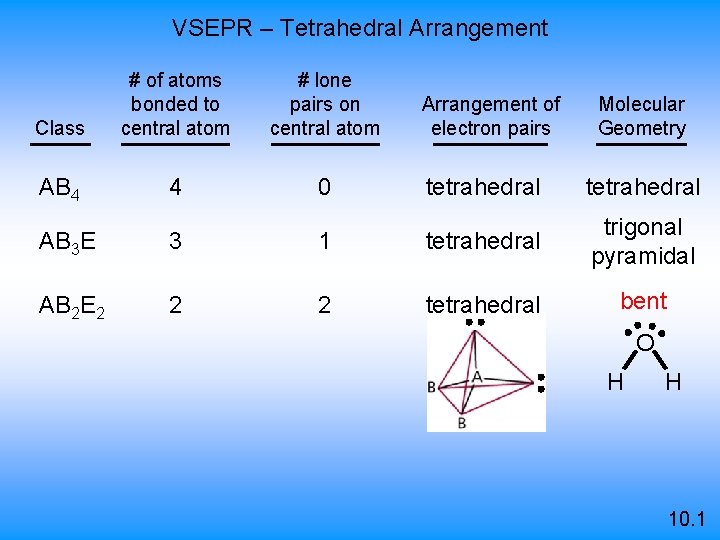
VSEPR – Tetrahedral Arrangement Class # of atoms bonded to central atom # lone pairs on central atom AB 4 4 0 Arrangement of electron pairs Molecular Geometry tetrahedral AB 3 E 3 1 tetrahedral trigonal pyramidal AB 2 E 2 2 2 tetrahedral bent O H H 10. 1

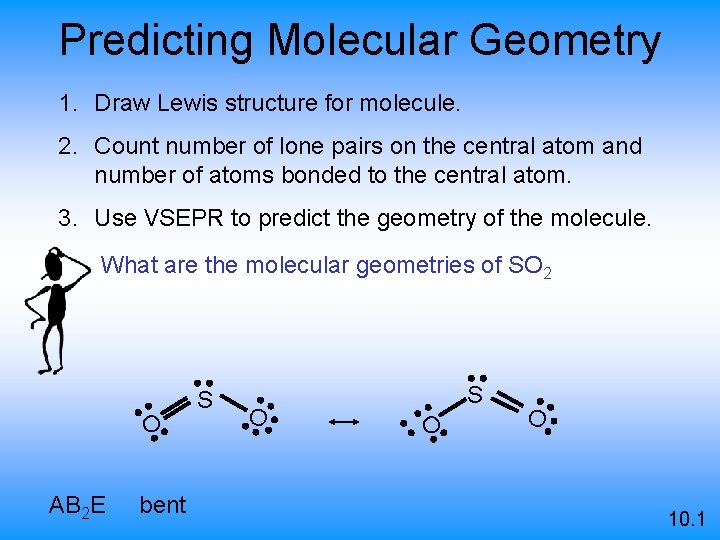
Predicting Molecular Geometry 1. Draw Lewis structure for molecule. 2. Count number of lone pairs on the central atom and number of atoms bonded to the central atom. 3. Use VSEPR to predict the geometry of the molecule. What are the molecular geometries of SO 2 S O AB 2 E bent O S O O 10. 1

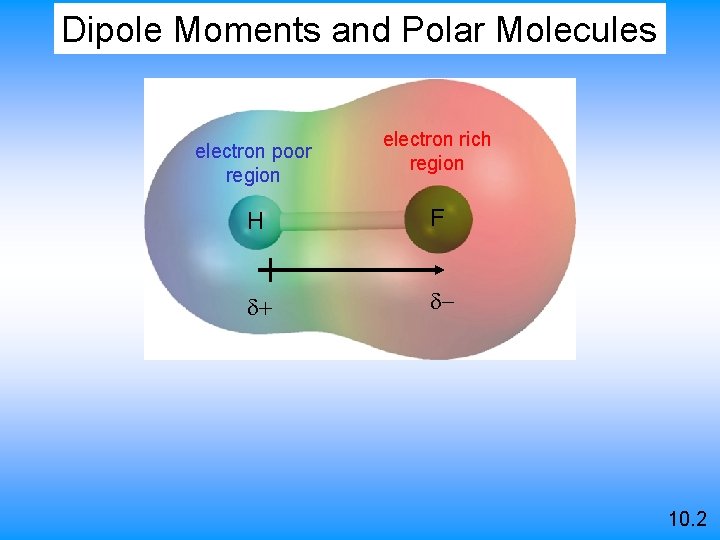
Dipole Moments and Polar Molecules electron poor region electron rich region H F d+ d- 10. 2

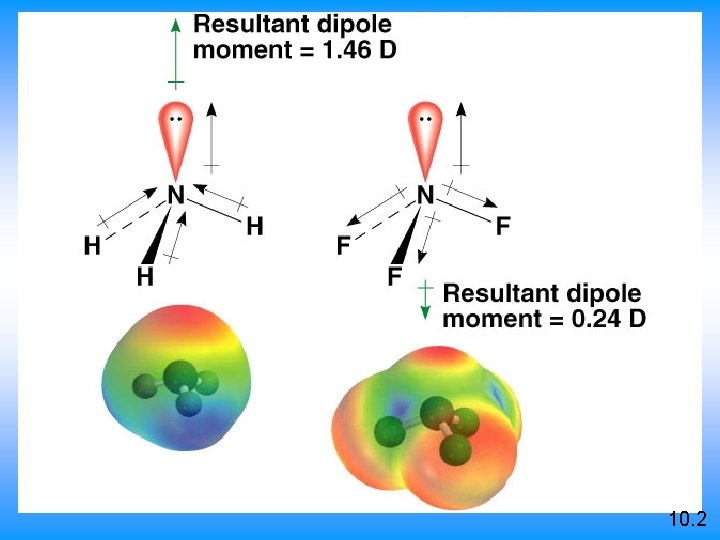
10. 2

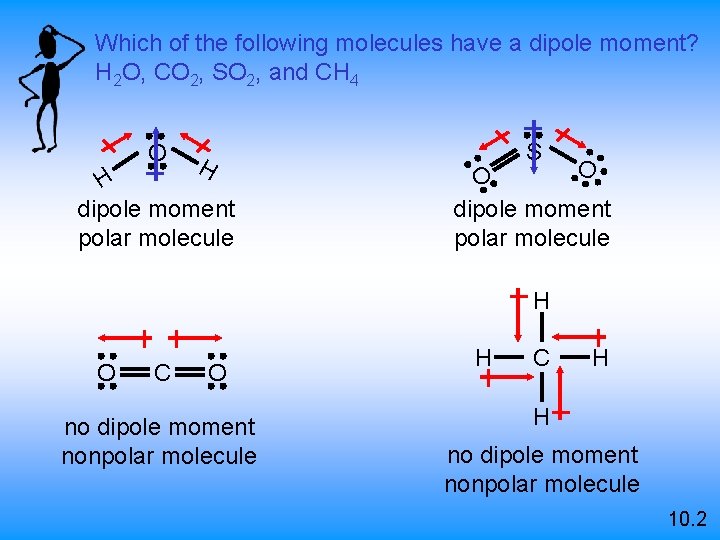
Which of the following molecules have a dipole moment? H 2 O, CO 2, SO 2, and CH 4 O H H dipole moment polar molecule S O O dipole moment polar molecule H O C O no dipole moment nonpolar molecule H C H H no dipole moment nonpolar molecule 10. 2

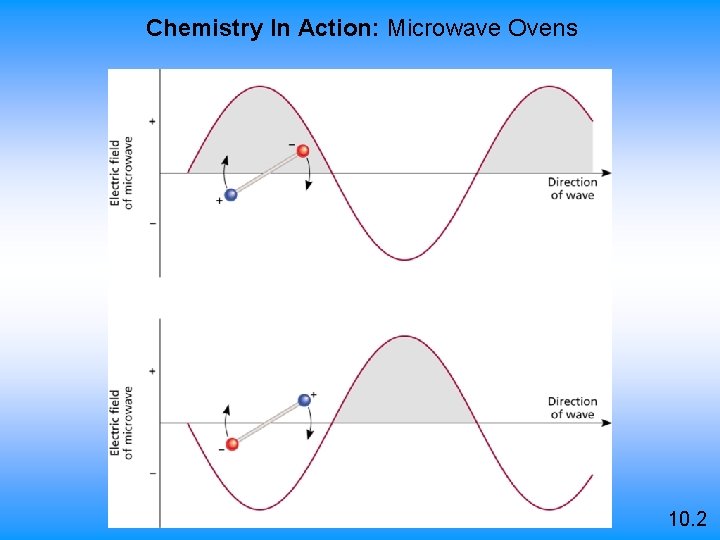
Chemistry In Action: Microwave Ovens 10. 2

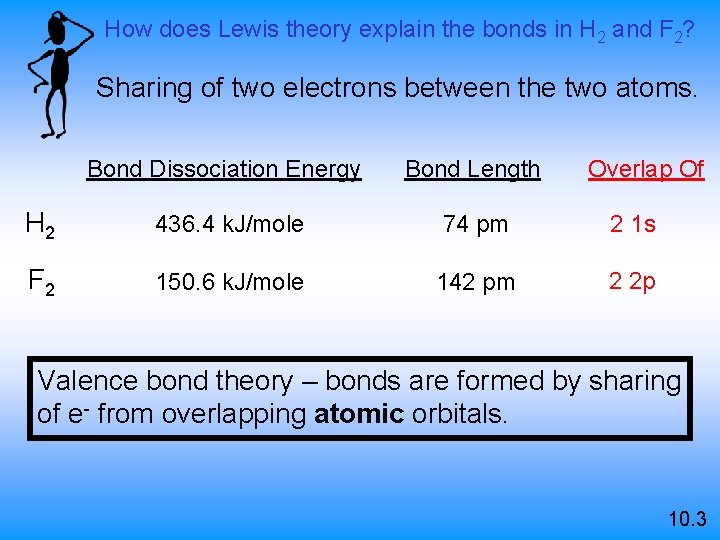
How does Lewis theory explain the bonds in H 2 and F 2? Sharing of two electrons between the two atoms. Overlap Of Bond Dissociation Energy Bond Length H 2 436. 4 k. J/mole 74 pm 2 1 s F 2 150. 6 k. J/mole 142 pm 2 2 p Valence bond theory – bonds are formed by sharing of e- from overlapping atomic orbitals. 10. 3