Vaccine adjuvants Viral vaccines in the medical practice











- Slides: 17

Vaccine adjuvants Viral vaccines in the medical practice 8 June 2010, Cluj-Napoca Kálmán Bartha Ph. D Zsuzsanna Pauliny MD bartha. kalman@oek. antsz. hu zsuzsanna. pauliny@oek. antsz. hu National Centre for Epidemiology Budapest, Hungary

Why we need adjuvants? l l Traditional vaccines based on attenuated live organisms already have them – their invasiveness provides efficient delivery to antigen-presenting cells and Various naturally occuring components of the pathogens stimulate the innate immune system The majority of recent vaccines represent highly purified subunit components of pathogens, they lack most of the features of the original pathogens, such as immunostimulatory components, and the ability to replicate and produce high level of antigens, Therefore, they are usually poorly immunogenic and need adjuvants to improve immunogenicity. 2

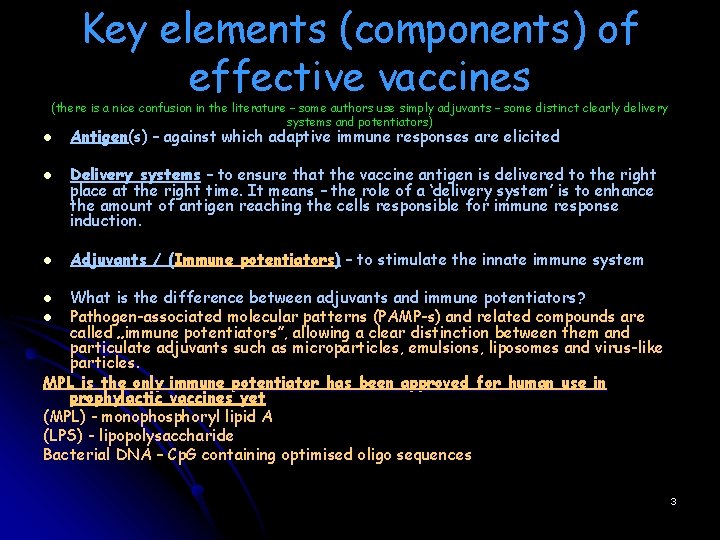
Key elements (components) of effective vaccines (there is a nice confusion in the literature – some authors use simply adjuvants – some distinct clearly delivery systems and potentiators) l Antigen(s) – against which adaptive immune responses are elicited l l Delivery systems – to ensure that the vaccine antigen is delivered to the right place at the right time. It means – the role of a ‘delivery system’ is to enhance the amount of antigen reaching the cells responsible for immune response induction. Adjuvants / (Immune potentiators) – to stimulate the innate immune system What is the difference between adjuvants and immune potentiators? l Pathogen-associated molecular patterns (PAMP-s) and related compounds are called „immune potentiators”, allowing a clear distinction between them and particulate adjuvants such as microparticles, emulsions, liposomes and virus-like particles. MPL is the only immune potentiator has been approved for human use in prophylactic vaccines yet (MPL) - monophosphoryl lipid A (LPS) - lipopolysaccharide Bacterial DNA – Cp. G containing optimised oligo sequences l 3

l l l The lipopolisaccharide (LPS) component of Gramnegative bacteria has been shown to act as a potent immune potentiator however, the profound toxicity and pyrogenicity of LPS prevents its use in humans. Alternatively, a chemically modified LPS derived from Salmonella minnesota R 595, called monophosphoryl lipid A (MPL), exhibits potent adjuvant activity with essentially no toxicity. MPL has been shown to be an effective immune potentiator for the induction of both humoral and cell -mediated immunity in which MPL can induce both Th 1 and Th 2 -type immune responses in the systemic and mucosal compartments of the immune system. 4

l l l Currently licensed adjuvants were developed using empirical methods. They are not optimal for many of the challenges in vaccination today. In particular, the historical emphasis on humoral immune responses has led to the development of adjuvants with the ability to enhance antibody response. As a consequence, most commonly used adjuvants are effective at elevating serum antibody titers, but do not elicit significant Th 1 responses or CTLs. 5

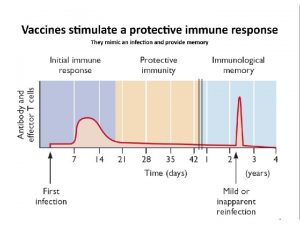
Innate/adaptive immune responses l l l The immune system has evolved two main functions: to react quickly (within minutes) to molecular patterns found in microbes, and to develop slowly (over days to weeks), precisely targeted specific adaptive immune responses. The faster acting innate immune responses provide a necessary first line of defense because of the relatively slow nature of adaptive immunity. In contrast, adaptive immunity uses selection and clonal expansion of immune cells harboring made-to-order somatically rearranged receptor genes (T- and B-cell receptors) recognising antigens from the pathogen, thereby providing specificity and long-lasting immunological memory. 6

Innate immune response l l Innate immune responses, among their many effects, lead to a rapid burst of inflammatory cytokines and activation of antigen-presenting cells (APCs) such as macrophages and dendritic cells. These nonclonal responses also lead to a conditioning of the immune system for subsequent development of specific adaptive immune responses. To distinguish pathogens from self-components, the innate immune system uses a wide variety of relatively invariable receptors that detect evolutionary conserved signatures from pathogens (pathogen-associated molecular patterns, PAMPs). 7

Immunological background I. l l l The addition of such microbial components to experimental vaccines leads to the development of robust and durable adaptive immune responses. The mechanism behind this potentiation of immune responses was not well understood as long as some of the pattern-recognition receptors (PRRs) involved in the innate immune responses to PAMPs were not identified. PRRs are differentially expressed on a wide variety of immune cells, including neutrophils, macrophages, dendritic cells, natural killer cells, B cells and in some nonimmune cells too, such as epithelial and endothelial cells. Engagement of PRRs leads to the activation of some of these cells and secretion of cytokines and chemokines, as well as maturation and migration of other cells. In tandem, this creates an inflammatory environment that leads to the establishment of the adaptive immune response. 8

Immunological background II. l l l PRRs consist of nonphagocytic receptors, such as Tolllike receptors (TLRs) and nucleotid-binding oligomerization domain (NOD) proteins, and receptors that induce phagocytosis, such as scavenger receptors, mannose receptors and β-glucan receptors. Receptors that induce phagocytosis are directly recognise ligands on the surface of pathogenic microbes and lead to their engulfment into phagocytic cells such as macrophages. Nonphagocytic receptors that recognise PAMPs extracellularly (certain TLRs) or intracellularly (NOD family of proteins) lead to an elaborate signal transduction cascade. 9 9

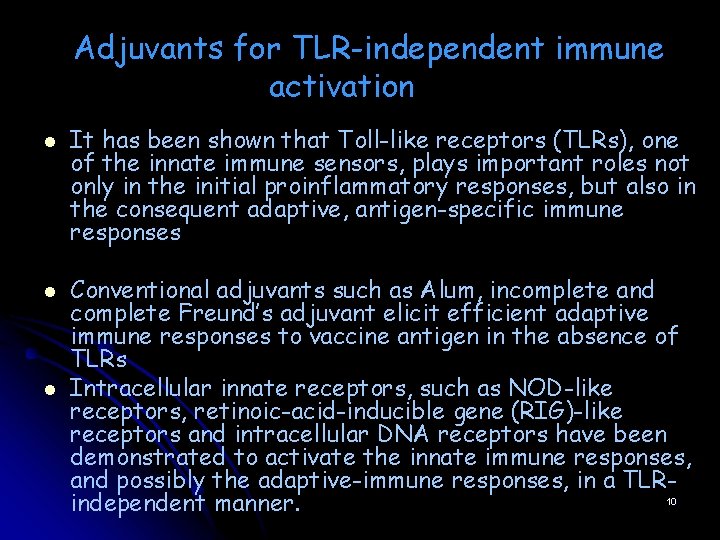
Adjuvants for TLR-independent immune activation l l l It has been shown that Toll-like receptors (TLRs), one of the innate immune sensors, plays important roles not only in the initial proinflammatory responses, but also in the consequent adaptive, antigen-specific immune responses Conventional adjuvants such as Alum, incomplete and complete Freund’s adjuvant elicit efficient adaptive immune responses to vaccine antigen in the absence of TLRs Intracellular innate receptors, such as NOD-like receptors, retinoic-acid-inducible gene (RIG)-like receptors and intracellular DNA receptors have been demonstrated to activate the innate immune responses, and possibly the adaptive-immune responses, in a TLR 10 independent manner.

Adjuvants for TLR-dependent immune activation l l l TLR ligands are promising candidates as vaccine adjuvants. In experimental vaccines TLR agonists are very potent adjuvants in capacity of activating cells expressing the TLR, in particular, dendritic cells (DCs), which are the key antigen presenting cells. The TLR 4 ligand LPS has been experimentally shown to be a potent adjuvant, although its extreme toxicity prevents its use in humans. The adjuvant effect of LPS is solely dependent on TLR 4 -mediated, My. D 88 dependent signaling. Efforts to eliminate the toxicity of lipid A led to the development of monophosphoryl lipid A (MPL) which is the only licensed new-generation TLR ligand vaccine adjuvant. MPL contains lipid A as a TLR 4 ligand. The dependency of TLR 4 on adjuvant effect of MPL was surprisingly minor, at least for antigenspecific antibody responses, suggesting that there are yet unknown TLR-independent adjuvant factors within the MPL compound. 11

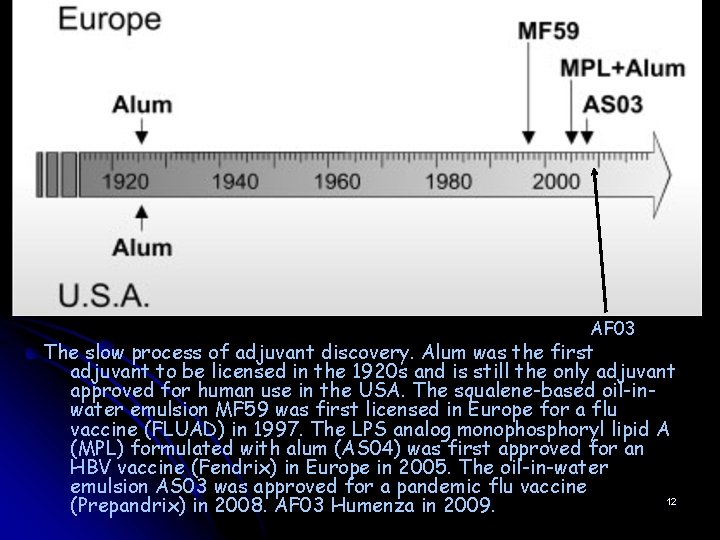
AF 03 The slow process of adjuvant discovery. Alum was the first adjuvant to be licensed in the 1920 s and is still the only adjuvant approved for human use in the USA. The squalene-based oil-inwater emulsion MF 59 was first licensed in Europe for a flu vaccine (FLUAD) in 1997. The LPS analog monophosphoryl lipid A (MPL) formulated with alum (AS 04) was first approved for an HBV vaccine (Fendrix) in Europe in 2005. The oil-in-water emulsion AS 03 was approved for a pandemic flu vaccine 12 (Prepandrix) in 2008. AF 03 Humenza in 2009.

13

Alum I. l l l Aluminium based mineral salts (generally called Alum) have been successfully used as adjuvants in licensed vaccines for many years. Alum typically induces Th 2 immune response. Although it has been shown to be safe and effective in traditional vaccines where eliciting antibody response is necessary, it is a weak adjuvant for protein subunits. Moreover, it fails to induce the Th 1 responses associated with the induction of gamma interferon and cytotoxic T lymphocytes (CTL) which are required to clear the body of intracellular viral infection. 14

15

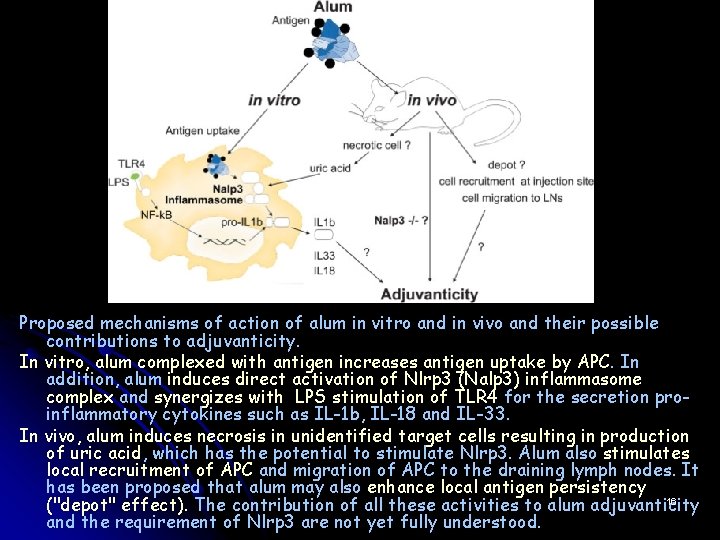
Proposed mechanisms of action of alum in vitro and in vivo and their possible contributions to adjuvanticity. In vitro, alum complexed with antigen increases antigen uptake by APC. In addition, alum induces direct activation of Nlrp 3 (Nalp 3) inflammasome complex and synergizes with LPS stimulation of TLR 4 for the secretion proinflammatory cytokines such as IL-1 b, IL-18 and IL-33. In vivo, alum induces necrosis in unidentified target cells resulting in production of uric acid, which has the potential to stimulate Nlrp 3. Alum also stimulates local recruitment of APC and migration of APC to the draining lymph nodes. It has been proposed that alum may also enhance local antigen persistency 16 ("depot" effect). The contribution of all these activities to alum adjuvanticity and the requirement of Nlrp 3 are not yet fully understood.

In summary – just alum It is very difficult to compare data obtained on the same adjuvant in different laboratories. In the case of alum, discrepancies may also arise from the multiple mechanisms of action, whether it is antigen delivery to APC or immunostimulation through Nlrp 3 activation. Some antigens may be contaminated by immunostimulatory molecules, therefore requiring only alum's antigen delivery function for an efficient adaptive response. On the other hand, other antigens may be easily internalized by APC despite the absence of alum but are poorly immunogenic and therefore may require Nlrp 3 -dependent alum-immunostimulatory activity. More work needs to be performed on inflammasome-deficient mice, using different immunization protocols and different formulations in parallel, in order to fully understand the contribution of the inflammasome to alum adjuvanticity. In summary, we are just beginning to understand the molecular mechanisms of alum, an adjuvant that has been used in humans for almost a century without knowing how it worked; however, standardized vaccination models are required to accurately address the differential contribution of each of the multiple mechanisms to adjuvanticity. 17
 Virulent
Virulent Glyphosate in vaccines
Glyphosate in vaccines Edible vaccines in pharmacognosy
Edible vaccines in pharmacognosy Stephanie seneff mit
Stephanie seneff mit Cancer vaccines
Cancer vaccines Tubertest négatif photo
Tubertest négatif photo Could vaccines breed viciousness
Could vaccines breed viciousness Edible vaccines pros and cons
Edible vaccines pros and cons Hep b vaccines
Hep b vaccines Shine skis encapsulated
Shine skis encapsulated Virulent
Virulent Www.cdc.gov/vaccines/schedules/index.html
Www.cdc.gov/vaccines/schedules/index.html History of vaccines pdf
History of vaccines pdf Global alliance for vaccines and immunization
Global alliance for vaccines and immunization Edible vaccines pros and cons
Edible vaccines pros and cons Brighton collaboration
Brighton collaboration Spacing out vaccines
Spacing out vaccines Aerochamber definition
Aerochamber definition