Update on the Mechanism of Action of Niacin





![Apo. A-I-Containing HDL Subpopulation Profiles of a Control and a CHD Patient pre [nm] Apo. A-I-Containing HDL Subpopulation Profiles of a Control and a CHD Patient pre [nm]](https://slidetodoc.com/presentation_image_h2/c35ebdf1ec200dc0660005b6ebff535b/image-8.jpg)




- Slides: 22

Update on the Mechanism of Action of Niacin for Use in High Risk Patients with CVD – Data in Humans, Monkeys, and Hamsters EJ Schaefer, MD Lipid Metabolism Laboratory, Human Nutrition Research Center on Aging at Tufts University, Boston, MA CRT, 2/6/12

Potential Conflicts of Interest > This research was funded by KOS, NIH, USDA Ø Ø Ø Research support in past 12 months from Abbott, Du. Pont, Resverlogix, Unilever Consultant in past 12 months for Arisaph, Du. Pont, Merck, Roche, Unilever Consultant and on Board of Directors of Boston Heart Laboratory (advanced heart disease risk assessment with clinical chemistry analysis, HDL mapping, genotyping, and sterol analysis)

Rudolph Altschul (1901 -1963): Niacin Arch Biochem 1955; 54: 558 -9.

Niacin n n “Niacin is the only available agent that significantly increases HDL-C concentrations” 1. “Among lipid-lowering agents, niacin is the most effective “HDL-raising drug” and effectively modifies all of the lipoprotein abnormalities associated with atherogenic dyslipidemia” 2. 1 Genest 2 Expert J, Frohlich J, Fodor G, Mc. Pherson R. CMAJ 2003; 168(9): 921 -4 Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults. September 2002. NIH publication 02 -5215.

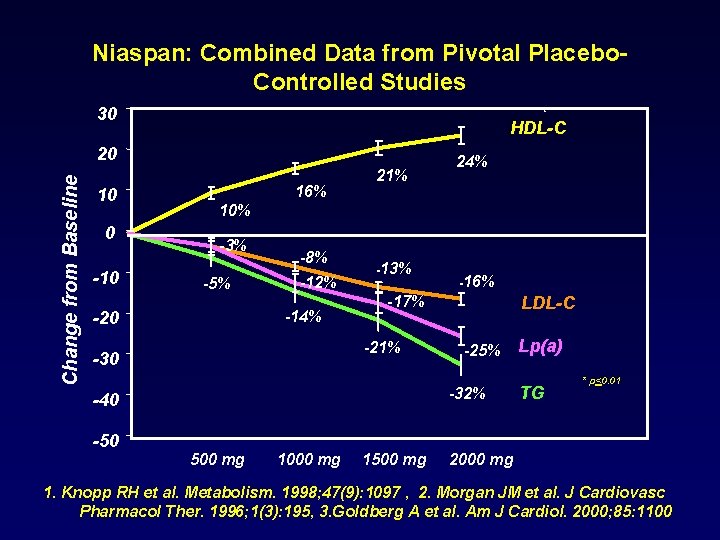
Niaspan: Combined Data from Pivotal Placebo. Controlled Studies 30 HDL-C Change from Baseline 20 10 0 -10 16% 21% 10% -3% -5% -8% -12% -14% -20 -13% -16% -17% -21% -30 LDL-C -25% -32% -40 -50 24% 500 mg 1000 mg 1500 mg Lp(a) TG * p<0. 01 2000 mg 1. Knopp RH et al. Metabolism. 1998; 47(9): 1097 , 2. Morgan JM et al. J Cardiovasc Pharmacol Ther. 1996; 1(3): 195, 3. Goldberg A et al. Am J Cardiol. 2000; 85: 1100

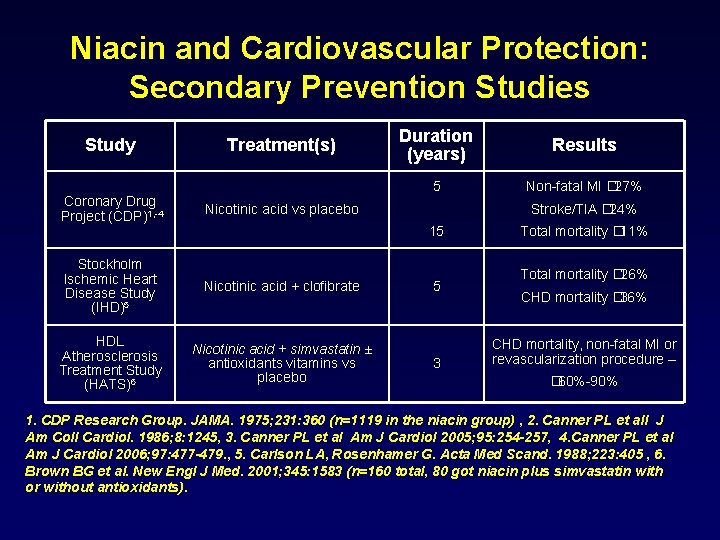
Niacin and Cardiovascular Protection: Secondary Prevention Studies Study Treatment(s) Coronary Drug Project (CDP)1, -4 Nicotinic acid vs placebo Stockholm Ischemic Heart Disease Study (IHD)5 Nicotinic acid + clofibrate HDL Atherosclerosis Treatment Study (HATS)6 Nicotinic acid + simvastatin ± antioxidants vitamins vs placebo Duration (years) Results 5 Non-fatal MI � 27% Stroke/TIA � 24% 15 5 3 Total mortality � 11% Total mortality � 26% CHD mortality � 36% CHD mortality, non-fatal MI or revascularization procedure – � 60%-90% 1. CDP Research Group. JAMA. 1975; 231: 360 (n=1119 in the niacin group) , 2. Canner PL et all J Am Coll Cardiol. 1986; 8: 1245, 3. Canner PL et al Am J Cardiol 2005; 95: 254 -257, 4. Canner PL et al Am J Cardiol 2006; 97: 477 -479. , 5. Carlson LA, Rosenhamer G. Acta Med Scand. 1988; 223: 405 , 6. Brown BG et al. New Engl J Med. 2001; 345: 1583 (n=160 total, 80 got niacin plus simvastatin with or without antioxidants).

AIM HIGH > Atherothrombosis Intervention in Metabolic Syndrome with Low HDL/ Hi TG and Impact on Global Health Outcomes > 3, 414 patients with vascular disease, low HDL/high TG randomized to simvastatin 40 -80 mg/day plus either 500 to 2, 000 mg of extended release niacin or placebo (each placebo capsule contained 50 mg of niacin, so that subjects on placebo received up to 200 mg/day of niacin). > In order to control for LDL-C levels – 22% of subjects in placebo group and 10% in niacin group were on ezetimibe 10 mg/day. LDL-C and HDL-C were 68 and 38 mg/dl on trial in control group and 63 and 42 mg/dl in Rx group – would predict 12. 5% event rate difference. No significance difference in any event rate parameters and trial was stopped early because of “futility. ” > However trial powered for 25% event rate reduction – therefore grossly underpowered. 25, 000 patient HPS 2 -THRIVE (reporting fall 2012 is powered to detect 12. 5% difference in event rate,
![Apo AIContaining HDL Subpopulation Profiles of a Control and a CHD Patient pre nm Apo. A-I-Containing HDL Subpopulation Profiles of a Control and a CHD Patient pre [nm]](https://slidetodoc.com/presentation_image_h2/c35ebdf1ec200dc0660005b6ebff535b/image-8.jpg)
Apo. A-I-Containing HDL Subpopulation Profiles of a Control and a CHD Patient pre [nm] 17. 0 9. 51 8. 16 7. 10 4. 66 Asztalos et al. Arterioscler Thromb Vasc Biol. 2003; 23: 847 -852; 2004; 24: 2181 -2187, 2005; 25: 2185 -2191, HATS Trial, Framingham Offspring Study, and the Veterans Affairs HDL Intervention Trial

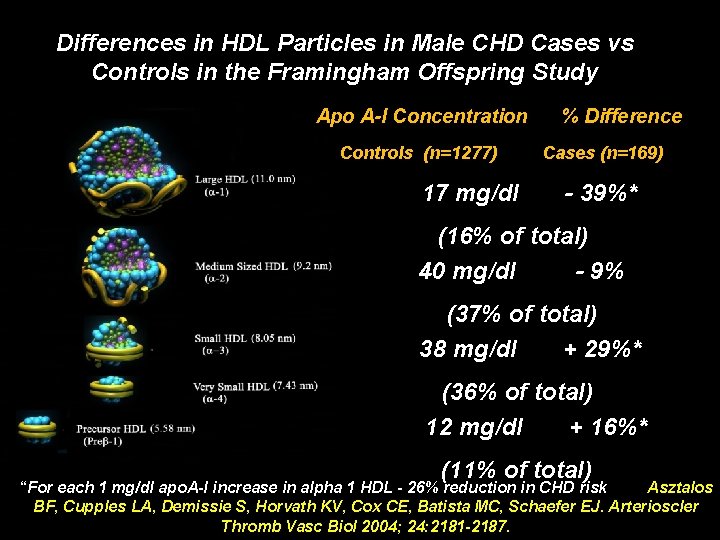
Differences in HDL Particles in Male CHD Cases vs Controls in the Framingham Offspring Study Apo A-I Concentration Controls (n=1277) 17 mg/dl % Difference Cases (n=169) - 39%* (16% of total) 40 mg/dl - 9% (37% of total) 38 mg/dl + 29%* (36% of total) 12 mg/dl + 16%* (11% of total) “For each 1 mg/dl apo. A-I increase in alpha 1 HDL - 26% reduction in CHD risk Asztalos BF, Cupples LA, Demissie S, Horvath KV, Cox CE, Batista MC, Schaefer EJ. Arterioscler Thromb Vasc Biol 2004; 24: 2181 -2187.

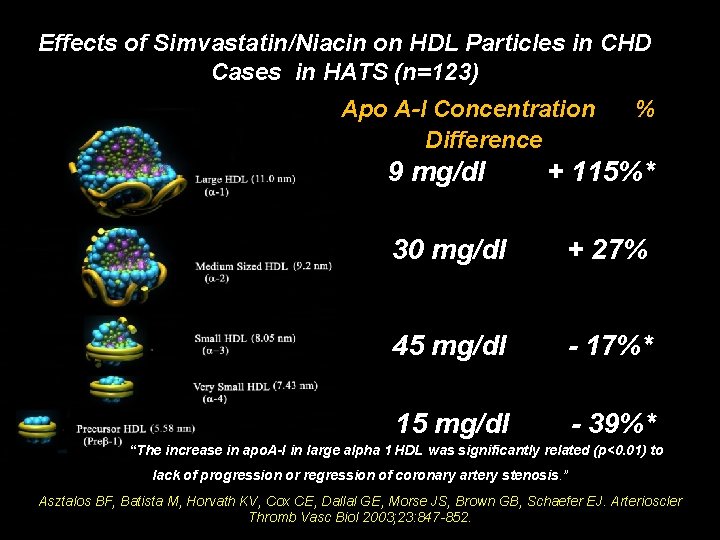
Effects of Simvastatin/Niacin on HDL Particles in CHD Cases in HATS (n=123) Apo A-I Concentration Difference 9 mg/dl % + 115%* 30 mg/dl + 27% 45 mg/dl - 17%* 15 mg/dl - 39%* “The increase in apo. A-I in large alpha 1 HDL was significantly related (p<0. 01) to lack of progression or regression of coronary artery stenosis. ” Asztalos BF, Batista M, Horvath KV, Cox CE, Dallal GE, Morse JS, Brown GB, Schaefer EJ. Arterioscler Thromb Vasc Biol 2003; 23: 847 -852.

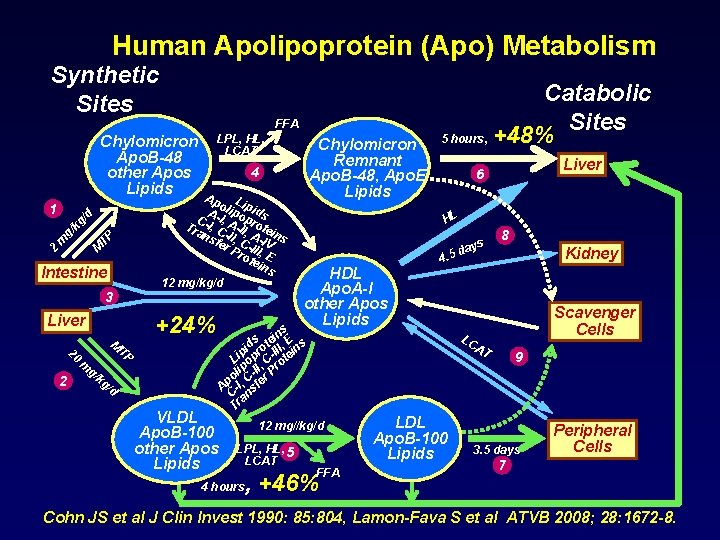
Human Apolipoprotein (Apo) Metabolism Synthetic Sites FFA Chylomicron Apo. B-48 other Apos Lipids kg M TP g/ m 2 Intestine 3 +24% Liver 20 2 M TP m g/ Chylomicron Remnant Apo. B-48, Apo. E Lipids 4 Ap L o ip A-I lipo ids p C , Tra -I, C A-II, rote ns -II, A-I ins fer C- V Pro III, E tei ns 12 mg/kg/d /d 1 LPL, HL, LCAT kg /d VLDL Apo. B-100 other Apos Lipids Catabolic Sites 5 hours, +48% HL s ay. 5 d HDL Apo. A-I other Apos Lipids Kidney Scavenger Cells LC AT 12 mg//kg/d 4 hours 8 4 s in E s e d t , s pi pro -III tein i L o , C o lip -II r Pr o C Ap -I, sfe C an Tr LPL, HL, 5 LCAT Liver 6 FFA , +46% LDL Apo. B-100 Lipids 9 3. 5 days Peripheral Cells 7 Cohn JS et al J Clin Invest 1990: 85: 804, Lamon-Fava S et al ATVB 2008; 28: 1672 -8.

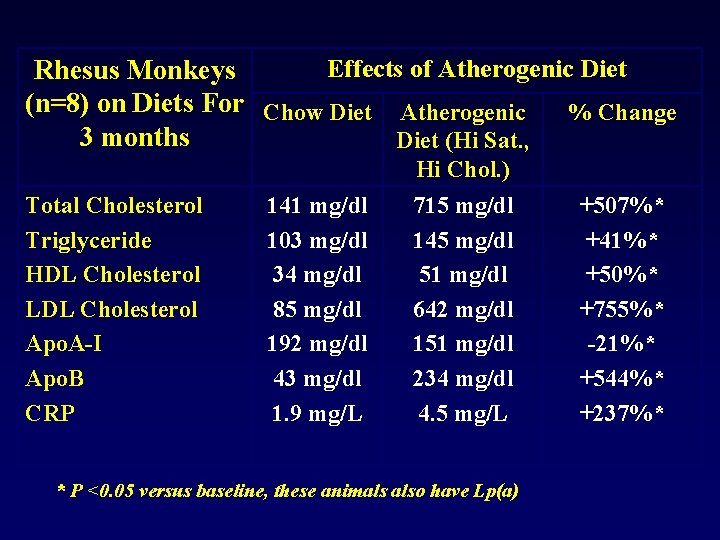
Effects of Atherogenic Diet Rhesus Monkeys (n=8) on Diets For Chow Diet Atherogenic % Change 3 months Diet (Hi Sat. , Hi Chol. ) Total Cholesterol Triglyceride HDL Cholesterol LDL Cholesterol Apo. A-I Apo. B CRP 141 mg/dl 103 mg/dl 34 mg/dl 85 mg/dl 192 mg/dl 43 mg/dl 1. 9 mg/L 715 mg/dl 145 mg/dl 51 mg/dl 642 mg/dl 151 mg/dl 234 mg/dl 4. 5 mg/L * P <0. 05 versus baseline, these animals also have Lp(a) +507%* +41%* +50%* +755%* -21%* +544%* +237%*

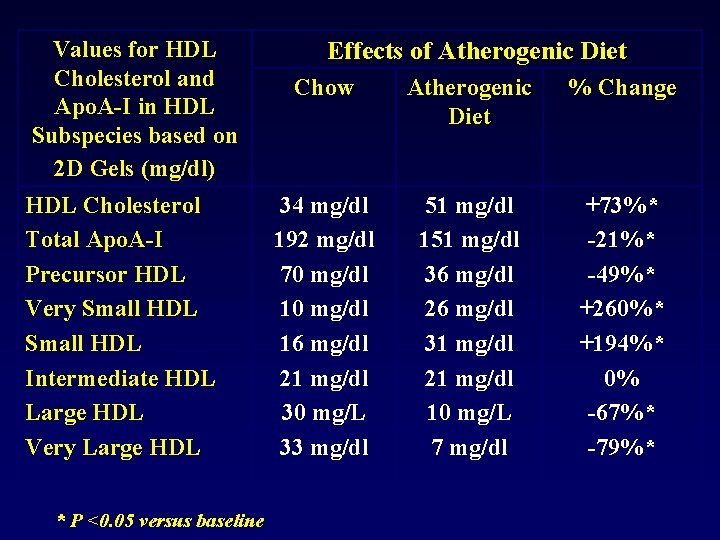
Values for HDL Cholesterol and Apo. A-I in HDL Subspecies based on 2 D Gels (mg/dl) HDL Cholesterol Total Apo. A-I Precursor HDL Very Small HDL Intermediate HDL Large HDL Very Large HDL * P <0. 05 versus baseline Effects of Atherogenic Diet Chow Atherogenic Diet % Change 34 mg/dl 192 mg/dl 70 mg/dl 16 mg/dl 21 mg/dl 30 mg/L 33 mg/dl 51 mg/dl 151 mg/dl 36 mg/dl 26 mg/dl 31 mg/dl 21 mg/dl 10 mg/L 7 mg/dl +73%* -21%* -49%* +260%* +194%* 0% -67%* -79%*

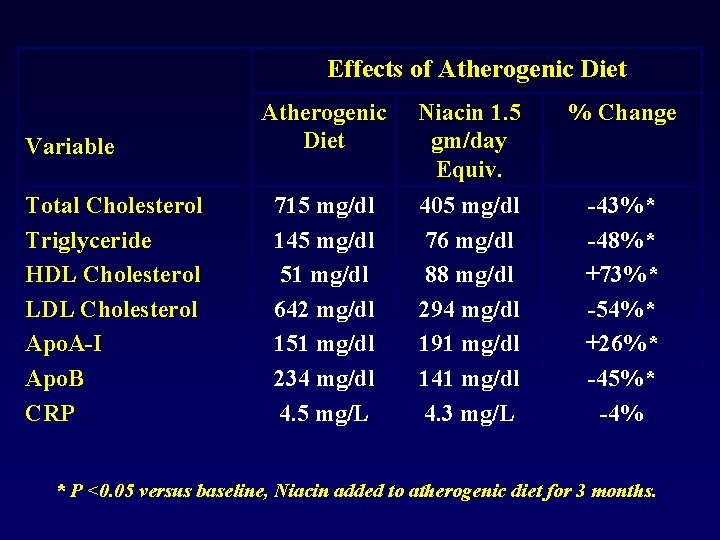
Effects of Atherogenic Diet Variable Total Cholesterol Triglyceride HDL Cholesterol LDL Cholesterol Apo. A-I Apo. B CRP Atherogenic Diet Niacin 1. 5 gm/day Equiv. % Change 715 mg/dl 145 mg/dl 51 mg/dl 642 mg/dl 151 mg/dl 234 mg/dl 4. 5 mg/L 405 mg/dl 76 mg/dl 88 mg/dl 294 mg/dl 191 mg/dl 141 mg/dl 4. 3 mg/L -43%* -48%* +73%* -54%* +26%* -45%* -4% * P <0. 05 versus baseline, Niacin added to atherogenic diet for 3 months.

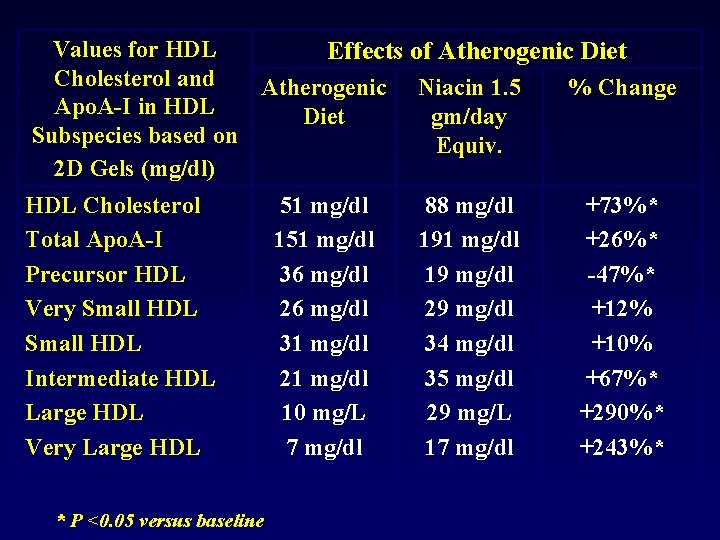
Values for HDL Effects of Atherogenic Diet Cholesterol and Atherogenic Niacin 1. 5 % Change Apo. A-I in HDL Diet gm/day Subspecies based on Equiv. 2 D Gels (mg/dl) HDL Cholesterol Total Apo. A-I Precursor HDL Very Small HDL Intermediate HDL Large HDL Very Large HDL * P <0. 05 versus baseline 51 mg/dl 151 mg/dl 36 mg/dl 26 mg/dl 31 mg/dl 21 mg/dl 10 mg/L 7 mg/dl 88 mg/dl 191 mg/dl 19 mg/dl 29 mg/dl 34 mg/dl 35 mg/dl 29 mg/L 17 mg/dl +73%* +26%* -47%* +12% +10% +67%* +290%* +243%*

Experiments in Hamsters (1) Background: ARI-3037 MO is a once-a-day structural analog of niacin that is currently in Phase I clinical trials for treatment of dyslipidemia. In several preclinical studies in multiple species, ARI-3037 MO did not induce the cutaneous flushing that is associated with high doses of niacin. Hypotheses: We hypothesized that ARI-3037 MO would display significant improvements in the lipoprotein profile of hamsters with diet-induced hyperlipidemia.

Experiments in Hamsters (2) Methods: Golden Syrian hamsters (n=30) (111 -120 g, 56 -61 days old) were fed a diet consisting of 11. 5% corn oil, 11. 5% coconut oil, 0. 5% cholesterol, and 0. 25% deoxycholate and were provided water containing 10% fructose for 20 days. After diet acclimation, animals remained on the atherogenic diet and were dosed P. O. with either vehicle (n=12), 1200 mg/kg/day niacin (n=9), or 1374 mg/kg/day ARI-3037 MO (n=9) for 18 days. At the end of the dosing period, hamsters were sacrificed via CO 2 asphyxiation, blood was collected via cardiac puncture, and tissues were harvested.

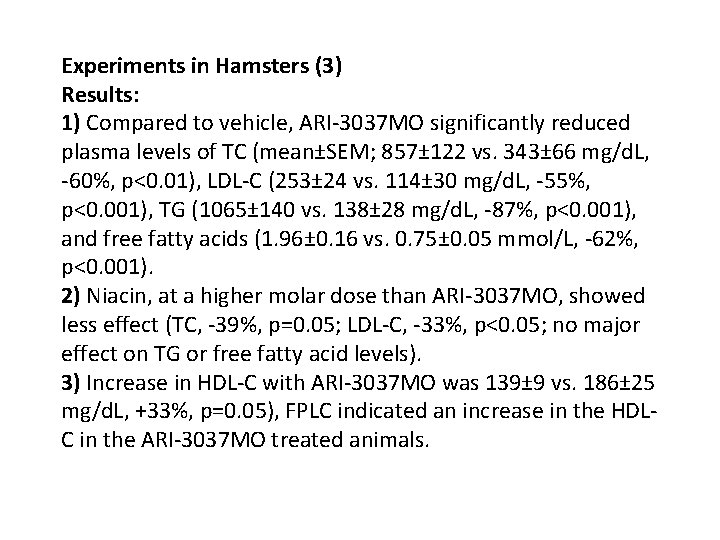
Experiments in Hamsters (3) Results: 1) Compared to vehicle, ARI-3037 MO significantly reduced plasma levels of TC (mean±SEM; 857± 122 vs. 343± 66 mg/d. L, -60%, p<0. 01), LDL-C (253± 24 vs. 114± 30 mg/d. L, -55%, p<0. 001), TG (1065± 140 vs. 138± 28 mg/d. L, -87%, p<0. 001), and free fatty acids (1. 96± 0. 16 vs. 0. 75± 0. 05 mmol/L, -62%, p<0. 001). 2) Niacin, at a higher molar dose than ARI-3037 MO, showed less effect (TC, -39%, p=0. 05; LDL-C, -33%, p<0. 05; no major effect on TG or free fatty acid levels). 3) Increase in HDL-C with ARI-3037 MO was 139± 9 vs. 186± 25 mg/d. L, +33%, p=0. 05), FPLC indicated an increase in the HDLC in the ARI-3037 MO treated animals.

Experiments in Hamsters (4) Results on Gene Expression: Relative to vehicle, ARI-3037 MO significantly increased hepatic m. RNA levels of apo. AI and ATP -Binding Cassette Transporter (ABC) A 1 m. RNA (+66%, p<0. 001 and +55%, p<0. 01, respectively). Conclusions: ARI-3037 MO was more effective than niacin at significantly reduced pro-atherogenic lipoproteins when compared to vehicle in the hyperlipidemic Golden Syrian hamster. In addition, ARI-3037 MO increased the proportion of HDL-C, possibly through liver apo. AI- and ABCA 1 -mediated mechanisms. Niacin had similar effects which are only seen at 4 hours instead of 24 hours.

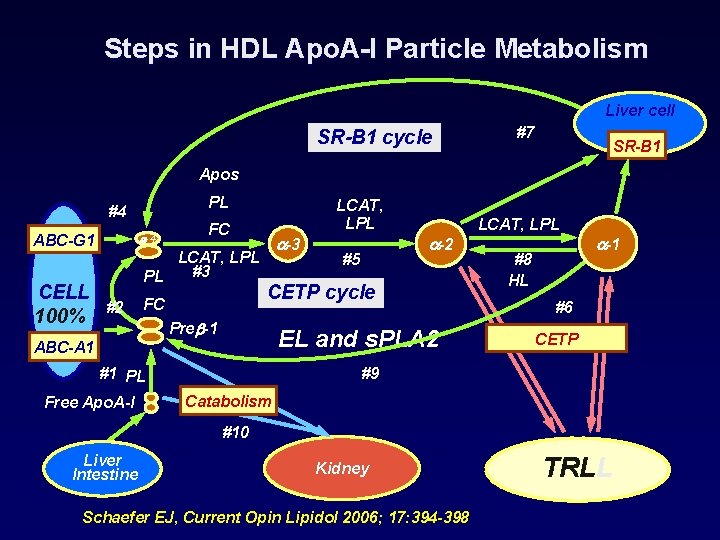
Steps in HDL Apo. A-I Particle Metabolism Liver cell SR-B 1 cycle #7 SR-B 1 Apos PL #4 ABC-G 1 CELL 100% -4 FC LCAT, LPL #3 PL #2 LCAT, LPL FC -3 #5 CETP cycle Pre -1 EL and s. PLA 2 ABC-A 1 #1 PL Free Apo. A-I -2 LCAT, LPL #8 HL -1 #6 CETP #9 Catabolism #10 Liver Intestine Kidney Schaefer EJ, Current Opin Lipidol 2006; 17: 394 -398 TRLL

Overall Effects of Niacin > In contrast to previous dogma niacin significantly enhances the fractional clearance of TRL apo. B-100 and modestly increases the secretion of HDL apo. A-I > Niacin has beneficial effects on fat by markedly increasing levels of anti-inflammatory adiponectin and its m. RNA levels > Niacin markedly increases liver ABCA 1 gene expression and moderately increases liver apo. A-I gene expression and causes the formation of very large HDL particles – it is unlikely that these effects are through the niacin receptor, since liver lacks this receptor > The overall data indicate that while statins inhibit cholesterol synthesis, niacin significantly enhances cellular cholesterol efflux and has beneficial effects in fat by upregulation of the anti-inflammatory adipokine adiponectin – therefore the combination is ideal for getting plaque regression.

THANK YOU FOR LISTENING
 Niacin absorption
Niacin absorption Function of niacin
Function of niacin What is an alternative of log based recovery
What is an alternative of log based recovery Class 3 antiarrhythmic drugs mechanism of action
Class 3 antiarrhythmic drugs mechanism of action Clomid mechanism of action
Clomid mechanism of action Thrombolytic drugs mechanism of action
Thrombolytic drugs mechanism of action Acetaminophen mechanism
Acetaminophen mechanism Dalacyn
Dalacyn Nesiritide mechanism of action
Nesiritide mechanism of action Pegfilgrastim mechanism of action
Pegfilgrastim mechanism of action Oral absorbable sulfonamides
Oral absorbable sulfonamides Glucagon mechanism of action
Glucagon mechanism of action Raprazole
Raprazole Mechanism insulin action
Mechanism insulin action Zidovudine mechanism of action
Zidovudine mechanism of action Antiplatelet mechanism of action
Antiplatelet mechanism of action Flucytosine mechanism of action
Flucytosine mechanism of action Antiduretics
Antiduretics Mechanism of action of asprin
Mechanism of action of asprin Theophylline mechanism of action
Theophylline mechanism of action Lactulose mechanism of action
Lactulose mechanism of action Theophylline mechanism of action
Theophylline mechanism of action Nitroglycerin mechanism of action
Nitroglycerin mechanism of action