An Update on Niacin Ernst J Schaefer MD












- Slides: 24

An Update on Niacin Ernst J. Schaefer, MD Distinguished University Professor, Tufts University School of Medicine, Director, Lipid Metabolism Laboratory & Cardiovascular Research Clinic, Boston, MA February 25 th, 2013

Ernst J. Schaefer, MD Consulting: Merck and Company, Inc. Grant Support: Abbott Laboratories and Du. Pont Honoraria: Arisaph Pharmaceuticals Stocks, Stock Options, other ownership interest: Boston Heart Laboratory

An Update on Niacin Ernst J. Schaefer, MD Distinguished University Professor Director, Lipid Metabolism Laboratory, Human Nutrition Research Center on Aging at Tufts University &Tufts University School of Medicine, Boston, MA USA Washington DC, February 25 th, 2013

Rudolph Altschul (1901 -1963): Niacin Arch Biochem 1955; 54: 558 -9.

Niacin n n “Niacin is the only available agent that significantly increases HDL-C concentrations” 1. “Among lipid-lowering agents, niacin is the most effective “HDL-raising drug” and effectively modifies all of the lipoprotein abnormalities associated with atherogenic dyslipidemia” 2. 1 Genest J, Frohlich J, Fodor G, Mc. Pherson R. CMAJ 2003; 168(9): 921 -4, 2 Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults, NIH 2002.

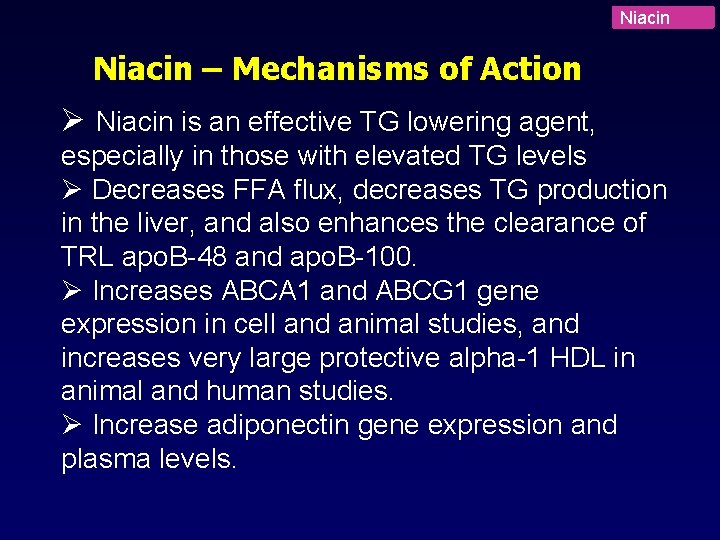
Niacin – Mechanisms of Action Ø Niacin is an effective TG lowering agent, especially in those with elevated TG levels Ø Decreases FFA flux, decreases TG production in the liver, and also enhances the clearance of TRL apo. B-48 and apo. B-100. Ø Increases ABCA 1 and ABCG 1 gene expression in cell and animal studies, and increases very large protective alpha-1 HDL in animal and human studies. Ø Increase adiponectin gene expression and plasma levels.

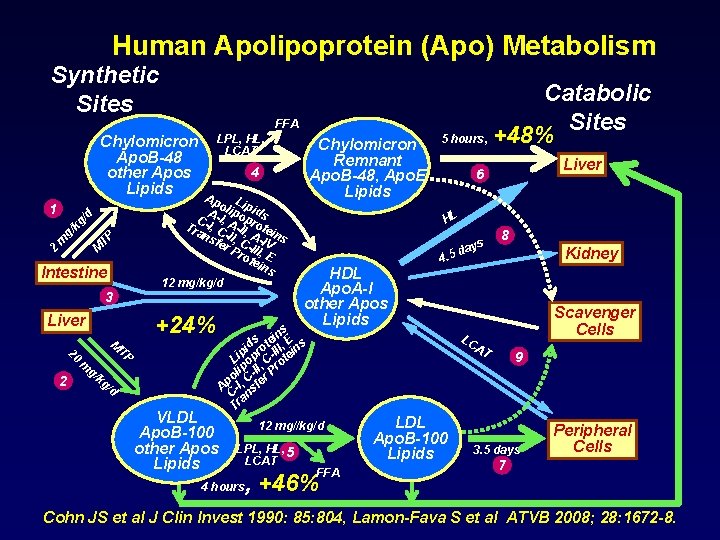
Human Apolipoprotein (Apo) Metabolism Synthetic Sites FFA Chylomicron Apo. B-48 other Apos Lipids kg M TP g/ m 2 Intestine 3 +24% Liver 20 2 M TP m g/ Chylomicron Remnant Apo. B-48, Apo. E Lipids 4 Ap L o ip A-I lipo ids p C , Tra -I, C A-II, rote ns -II, A-I ins fer C- V Pro III, E tei ns 12 mg/kg/d /d 1 LPL, HL, LCAT kg /d VLDL Apo. B-100 other Apos Lipids Catabolic Sites 5 hours, +48% HL s ay. 5 d HDL Apo. A-I other Apos Lipids Kidney Scavenger Cells LC AT 12 mg//kg/d 4 hours 8 4 s in E s e d t , s pi pro -III tein i L o , C o lip -II r Pr o C Ap -I, sfe C an Tr LPL, HL, 5 LCAT Liver 6 FFA , +46% LDL Apo. B-100 Lipids 9 3. 5 days Peripheral Cells 7 Cohn JS et al J Clin Invest 1990: 85: 804, Lamon-Fava S et al ATVB 2008; 28: 1672 -8.

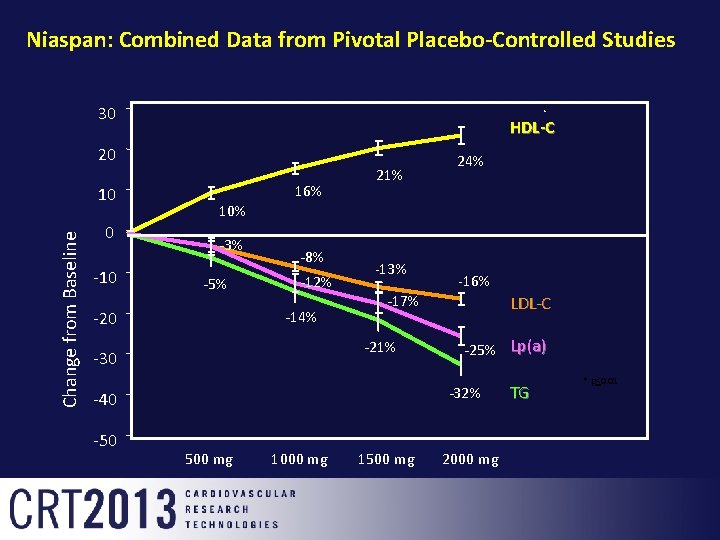
Niaspan: Combined Data from Pivotal Placebo-Controlled Studies 30 HDL-C 20 Change from Baseline 10 0 -10 10% -3% -5% 16% -8% -12% -14% -20 21% -13% LDL-C -25% Lp(a) -32% -40 -50 -16% -17% -21% -30 24% 500 mg 1000 mg 1500 mg 2000 mg TG * p<0. 01

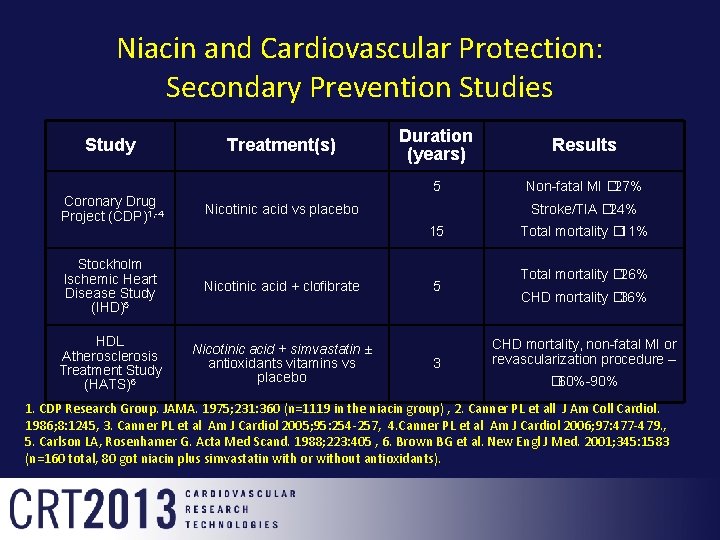
Niacin and Cardiovascular Protection: Secondary Prevention Studies Study Treatment(s) Coronary Drug Project (CDP)1, -4 Nicotinic acid vs placebo Stockholm Ischemic Heart Disease Study (IHD)5 Nicotinic acid + clofibrate HDL Atherosclerosis Treatment Study (HATS)6 Nicotinic acid + simvastatin ± antioxidants vitamins vs placebo Duration (years) Results 5 Non-fatal MI � 27% Stroke/TIA � 24% 15 5 3 Total mortality � 11% Total mortality � 26% CHD mortality � 36% CHD mortality, non-fatal MI or revascularization procedure – � 60%-90% 1. CDP Research Group. JAMA. 1975; 231: 360 (n=1119 in the niacin group) , 2. Canner PL et all J Am Coll Cardiol. 1986; 8: 1245, 3. Canner PL et al Am J Cardiol 2005; 95: 254 -257, 4. Canner PL et al Am J Cardiol 2006; 97: 477 -479. , 5. Carlson LA, Rosenhamer G. Acta Med Scand. 1988; 223: 405 , 6. Brown BG et al. New Engl J Med. 2001; 345: 1583 (n=160 total, 80 got niacin plus simvastatin with or without antioxidants).

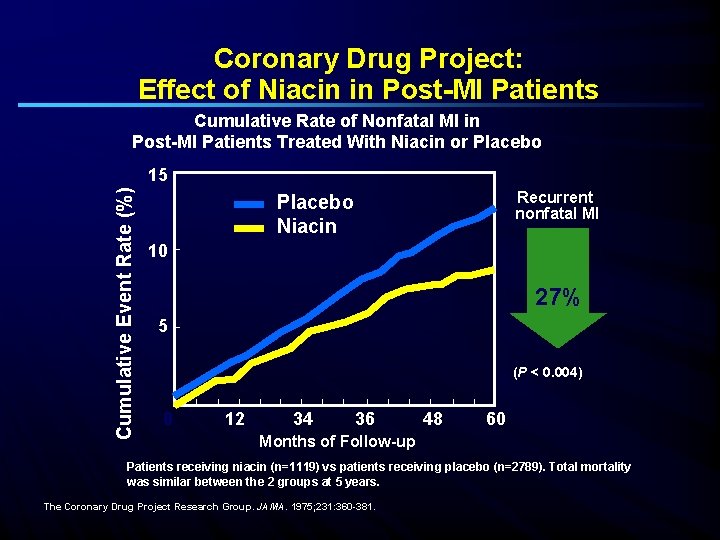
Coronary Drug Project: Effect of Niacin in Post-MI Patients Cumulative Rate of Nonfatal MI in Post-MI Patients Treated With Niacin or Placebo Cumulative Event Rate (%) 15 Recurrent nonfatal MI Placebo Niacin 10 27% 5 (P < 0. 004) 0 12 34 36 48 60 Months of Follow-up Patients receiving niacin (n=1119) vs patients receiving placebo (n=2789). Total mortality was similar between the 2 groups at 5 years. The Coronary Drug Project Research Group. JAMA. 1975; 231: 360 -381.

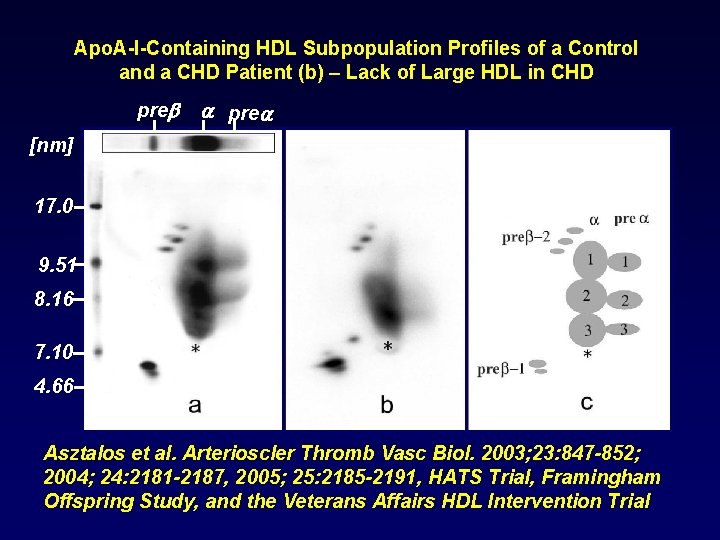
Apo. A-I-Containing HDL Subpopulation Profiles of a Control and a CHD Patient (b) – Lack of Large HDL in CHD pre [nm] 17. 0 9. 51 8. 16 7. 10 4. 66 Asztalos et al. Arterioscler Thromb Vasc Biol. 2003; 23: 847 -852; 2004; 24: 2181 -2187, 2005; 25: 2185 -2191, HATS Trial, Framingham Offspring Study, and the Veterans Affairs HDL Intervention Trial

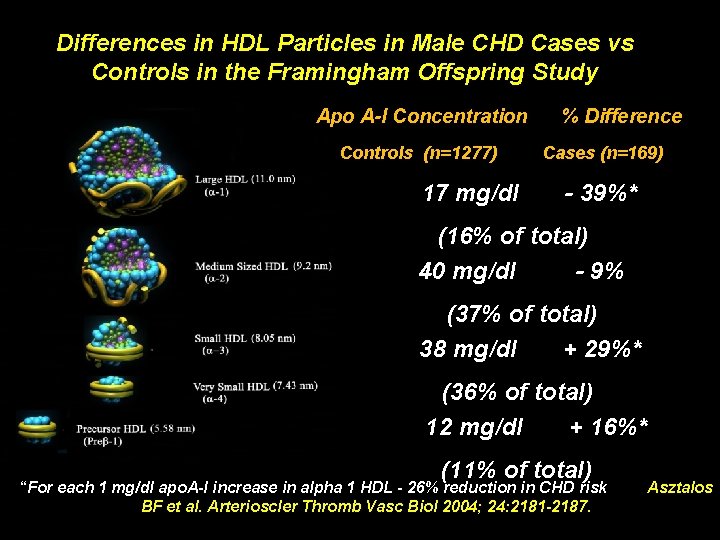
Differences in HDL Particles in Male CHD Cases vs Controls in the Framingham Offspring Study Apo A-I Concentration Controls (n=1277) 17 mg/dl % Difference Cases (n=169) - 39%* (16% of total) 40 mg/dl - 9% (37% of total) 38 mg/dl + 29%* (36% of total) 12 mg/dl + 16%* (11% of total) “For each 1 mg/dl apo. A-I increase in alpha 1 HDL - 26% reduction in CHD risk BF et al. Arterioscler Thromb Vasc Biol 2004; 24: 2181 -2187. Asztalos

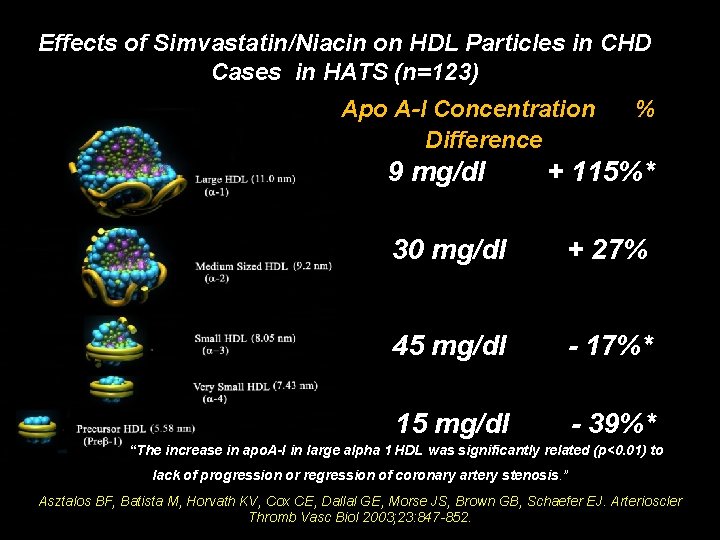
Effects of Simvastatin/Niacin on HDL Particles in CHD Cases in HATS (n=123) Apo A-I Concentration Difference 9 mg/dl % + 115%* 30 mg/dl + 27% 45 mg/dl - 17%* 15 mg/dl - 39%* “The increase in apo. A-I in large alpha 1 HDL was significantly related (p<0. 01) to lack of progression or regression of coronary artery stenosis. ” Asztalos BF, Batista M, Horvath KV, Cox CE, Dallal GE, Morse JS, Brown GB, Schaefer EJ. Arterioscler Thromb Vasc Biol 2003; 23: 847 -852.

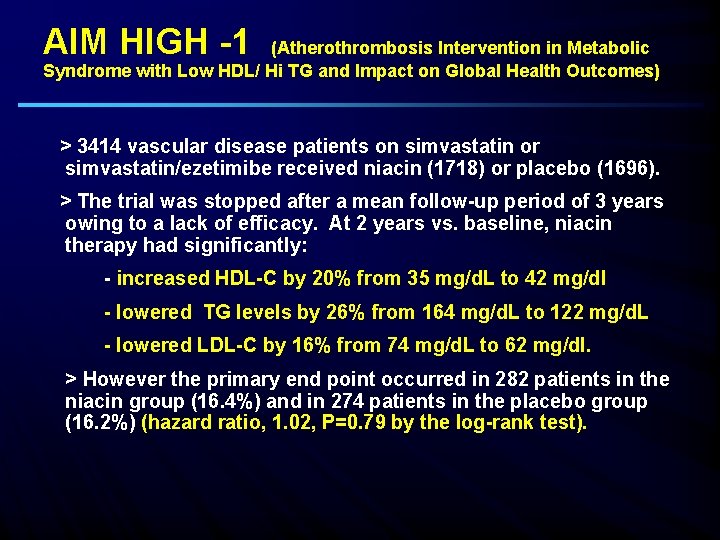
AIM HIGH -1 (Atherothrombosis Intervention in Metabolic Syndrome with Low HDL/ Hi TG and Impact on Global Health Outcomes) > 3414 vascular disease patients on simvastatin or simvastatin/ezetimibe received niacin (1718) or placebo (1696). > The trial was stopped after a mean follow-up period of 3 years owing to a lack of efficacy. At 2 years vs. baseline, niacin therapy had significantly: - increased HDL-C by 20% from 35 mg/d. L to 42 mg/dl - lowered TG levels by 26% from 164 mg/d. L to 122 mg/d. L - lowered LDL-C by 16% from 74 mg/d. L to 62 mg/dl. > However the primary end point occurred in 282 patients in the niacin group (16. 4%) and in 274 patients in the placebo group (16. 2%) (hazard ratio, 1. 02, P=0. 79 by the log-rank test).

AIM HIGH -2 (Atherothrombosis Intervention in Metabolic Syndrome with Low HDL/ Hi TG and Impact on Global Health Outcomes) Ø Each placebo capsule contained 50 mg of niacin, so subjects on placebo received up to 200 mg/day of niacin. Ø In order to control for LDL-C levels, 22% of subjects in the placebo group and 10% in the niacin group were on ezetimibe 10 mg/day. Ø LDL-C and HDL-C were 68 and 38 mg/dl on trial in the control group and 63 (-7%) and 42 (+9%) mg/dl in the treatment group on trial. Ø The study was powered to see a 25% risk reduction in the primary endpoint. Boden W et al N Engl J Med. 2011; 365: 2255 -67.

AIM HIGH – Why Did the Study Fail? All of the Below May Have Contributed. Ø The study was underpowered. Ø The study was stopped too early. Ø The placebo group got low dose niacin which may have had an effect, and also had beneficial changes relative to baseline. Ø Twice as many patients in the placebo group received ezetimibe as compared to the treatment group. Ø Mean LDL-C at 63 mg/d. L was only 7% lower in the treatment group than in the placebo group. Raising HDL-C when LDL-C is < 70 mg/d. L may not be effective in CHD risk reduction. Ø Mean HDL-C at 42 mg/d. L was only 9% higher in the treatment group than in the placebo group.

AIM HIGH – Subgroup Analysis Ø Post-hoc subgroup analysis of AIM-HIGH Ø CHD subjects with TG > 200 mg/d. L and HDL-C < 32 mg/d. L Ø This group had the highest risk of recurrent CHD events in the placebo group (statin alone or statin plus ezetimibe) Ø Moreover in this subgroup those who received niacin had a relative risk of 0. 63 (p=0. 017) versus placebo group. Guyton J et al AHA November 7 th, 2012

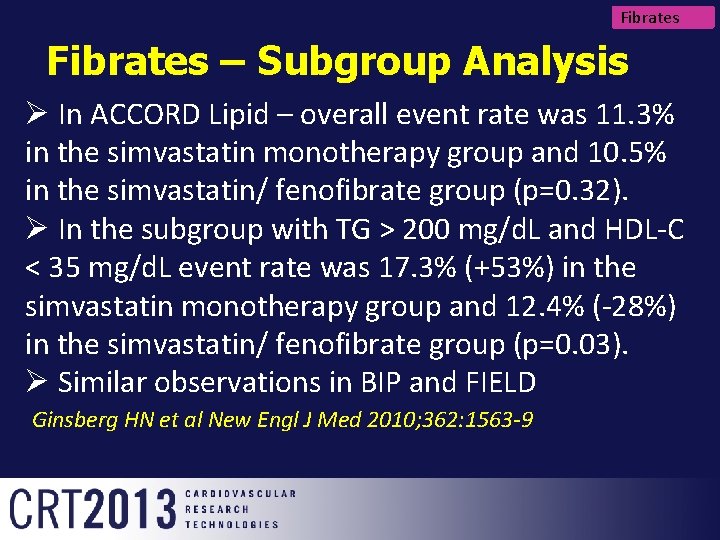
Fibrates – Subgroup Analysis Ø In ACCORD Lipid – overall event rate was 11. 3% in the simvastatin monotherapy group and 10. 5% in the simvastatin/ fenofibrate group (p=0. 32). Ø In the subgroup with TG > 200 mg/d. L and HDL-C < 35 mg/d. L event rate was 17. 3% (+53%) in the simvastatin monotherapy group and 12. 4% (-28%) in the simvastatin/ fenofibrate group (p=0. 03). Ø Similar observations in BIP and FIELD Ginsberg HN et al New Engl J Med 2010; 362: 1563 -9

HPS 2 -THRIVE: Heart Protection Study 2: Treatment of HDL to Reduce the Incidence of Vascular Events Ø Presented by Prof. Jane Armitage, Oxford, PI, ESC/ 9/2012 Ø 25, 673 high-risk patients with occlusive arterial disease from China, Scandinavia and UK randomized into study Ø Randomized blinded comparison: ER niacin/ laropiprant (ERN/LRPT) 2 g daily versus placebo Ø Primary end point: Major vascular events after median follow -up of 4 years Ø Pre-specified safety analyses: Median follow-up of 3. 4 years (to January 2012) Ø Background LDL-lowering therapy with: Simvastatin 40 mg (+/- ezetimibe 10 mg) daily

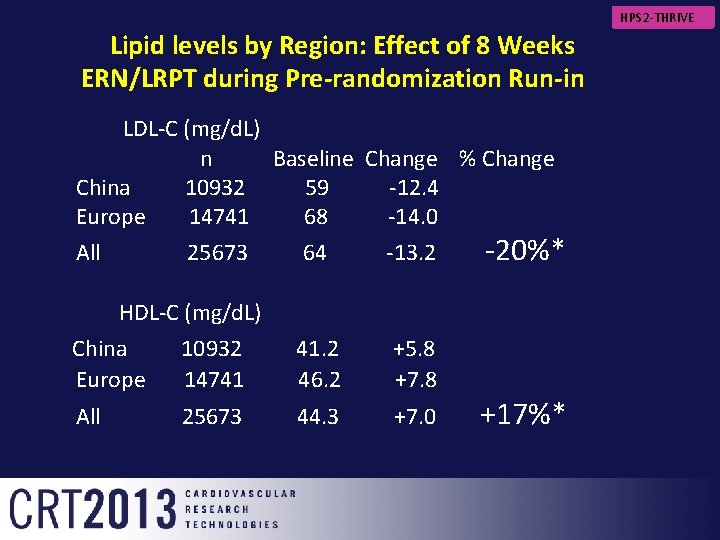
HPS 2 -THRIVE Lipid levels by Region: Effect of 8 Weeks ERN/LRPT during Pre-randomization Run-in LDL-C (mg/d. L) n Baseline Change % Change China 10932 59 -12. 4 Europe 14741 68 -14. 0 All 25673 64 -13. 2 -20%* HDL-C (mg/d. L) China 10932 41. 2 +5. 8 Europe 14741 46. 2 +7. 8 All 25673 44. 3 +7. 0 +17%*

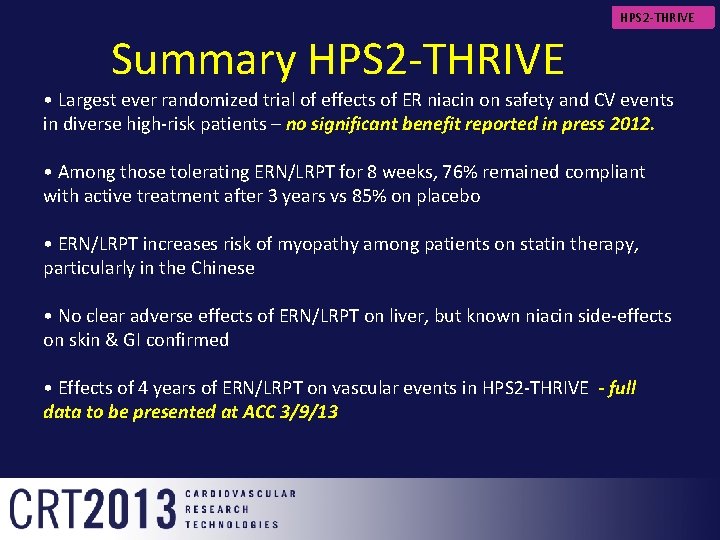
HPS 2 -THRIVE Summary HPS 2 -THRIVE • Largest ever randomized trial of effects of ER niacin on safety and CV events in diverse high-risk patients – no significant benefit reported in press 2012. • Among those tolerating ERN/LRPT for 8 weeks, 76% remained compliant with active treatment after 3 years vs 85% on placebo • ERN/LRPT increases risk of myopathy among patients on statin therapy, particularly in the Chinese • No clear adverse effects of ERN/LRPT on liver, but known niacin side-effects on skin & GI confirmed • Effects of 4 years of ERN/LRPT on vascular events in HPS 2 -THRIVE - full data to be presented at ACC 3/9/13

Potential Adverse Effects of Laropiprant Ø The effects of niacin on lipids are independent of GPR 109 A, but not the flushing effects, which are due to cyclooxygenase (COX)-1 mediated production of prostaglandin (PG) D 2, followed by the COX-2 mediated production of PGE 2 (1). Ø PGD 2 acts by binding to the prostanoid receptors DP 1 and DP 2. Laropiprant decreases the flushing induced by niacin by inhibiting the DP 1 receptor. Ø DP 1 depletion in mice leads to increased atherosclerosis, thrombosis, aneurysm formation, and hypertensive response to angiotensinogen II (2). Ø Niacin inhibits atherosclerosis in mice via GPR 109 A by inducing ABCG 1 dependent macrophage cholesterol efflux, and by inhibiting MIP-1 dependent macrophage recruitment (1). Ø Laropiprant, like COX-2 inhibitors, may cause increased CVD risk. Low dose aspirin might be a far better alternative to lower flushing (2). (1) Lukasova M et al J Clin Invest 2010; 121: 1163 -73. (2) Song WL et al. J Clin Invest 2012; 122: 1459 -68.

Niacin Score Card + Ø Coronary Drug Project Ø Stockholm Ischemic Heart Disease Study + Ø HATS, Other Angiographic Studies + Ø AIM HIGH – except subgroup TG > 200 mg/d. L and HDL-C < 32 mg/d. L -37% RR Ø HPS 2 -THRIVE – subgroup analysis? Ø More data from Dr. Armitage at ACC 3/9/13 Late Breaking Trials Niacin

Thank you for your attention