Units of Counting Dozen 12 Pair 2 Gross







- Slides: 12


Units of Counting § § Dozen 12 Pair 2 Gross 144 Ream 500


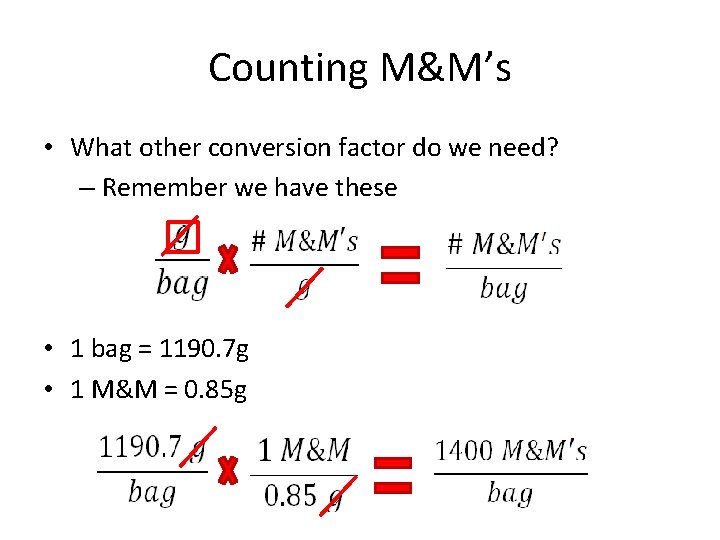
Counting M&M’s • Example 1: How many M&M’s are in one party-sized bag? – Given on the bag… • 1 bag = 1190. 7 g – We want… • # M&M’s per bag

Counting M&M’s • What other conversion factor do we need? – Remember we have these • 1 bag = 1190. 7 g • 1 M&M = 0. 85 g

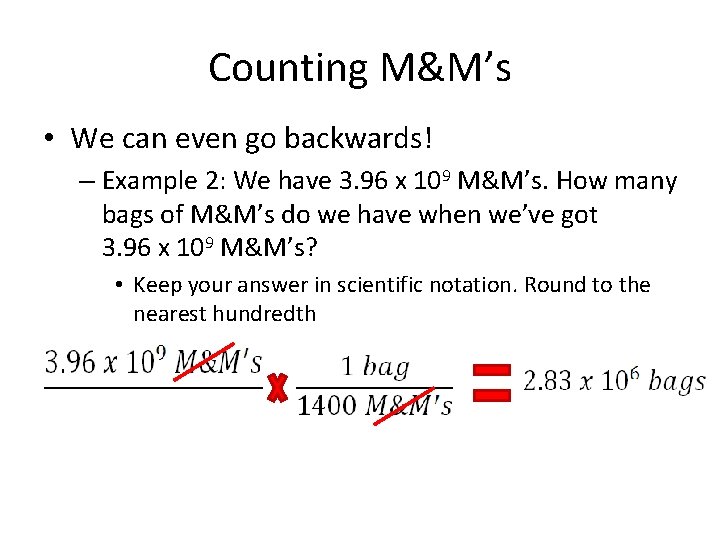
Counting M&M’s • We can even go backwards! – Example 2: We have 3. 96 x 109 M&M’s. How many bags of M&M’s do we have when we’ve got 3. 96 x 109 M&M’s? • Keep your answer in scientific notation. Round to the nearest hundredth

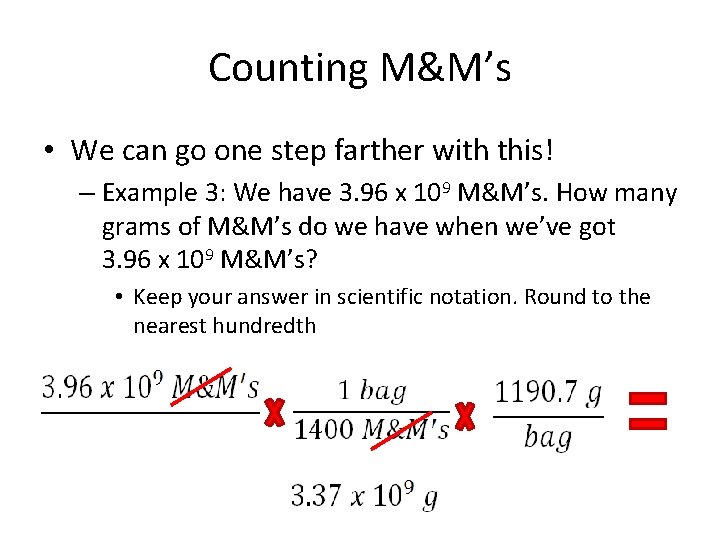
Counting M&M’s • We can go one step farther with this! – Example 3: We have 3. 96 x 109 M&M’s. How many grams of M&M’s do we have when we’ve got 3. 96 x 109 M&M’s? • Keep your answer in scientific notation. Round to the nearest hundredth
Counting M&M’s as an analogy • We counted 1400 M&M’s without physically counting them. – We counted M&M’s by mass. • That is what chemists do with the number of particles in chemicals. – Chemists count particles by mass.

Units of Counting § § § Dozen 12 Pair 2 Gross 144 Ream 500 Party-sized Bag 1400 M&Ms Moles (abbreviated: mol) 6. 022 x 1023 particles Simply a counting unit

Avogadro’s Number • Named after Italian scientist • Avogadro DID NOT discover Avogadro’s number – His work paved the way to the discovery • His work inspired other scientists to experiment on pure carbon-12 – They found that there are 6. 0221367 x 1023 atoms in exactly 12. 0000 g of pure carbon-12

Avogadro’s Number • Definition: the number of particles in a mole. • This number is 6. 022 x 1023 – Written as 6. 022 x 1023 particles/mol • In the M&M analogy: 1400 M&Ms/bag

Molar Mass • • The mass of one mole Units are g/mol In the M&M analogy g/bag Round the atomic mass from the periodic table to the second decimal place
 Pair dozen gross ream
Pair dozen gross ream Adipic acid empirical formula
Adipic acid empirical formula Why is it useful to use units like a dozen or a ream
Why is it useful to use units like a dozen or a ream Tabel diskon faktor
Tabel diskon faktor Python unordered pair
Python unordered pair Dof of screw pair
Dof of screw pair Speywood units vs botox units
Speywood units vs botox units Variable costing income statement
Variable costing income statement Mass of a dozen eggs
Mass of a dozen eggs Give me a dozen healthy infants well-formed meaning
Give me a dozen healthy infants well-formed meaning Msha dirty dozen
Msha dirty dozen Mass of a dozen eggs
Mass of a dozen eggs Dozen font
Dozen font























