Counting units such as dozen or gross are



- Slides: 9

Counting units such as dozen or gross are commonly used to deal with large quantities of items. No matter what we are counting, a dozen means 12, whether we are counting oranges, pencils, or even elephants.

Review in your text – a mole • Pages 83 – 87 definitions and practice • Avogadro’s number, rounded to 6. 02 x 10 23 is used to describe the amount of a substance so that chemists can determine the molar mass in one mole. Using the average atomic mass for each atom we can use dimensional analysis to determine the mass of 1 mole in grams for any substance. CHECK FOR UNDERSTANDING : COMPLETE PRACTICE PROBLEMS PG 85

What is a mole? • The mole is a counting unit used by chemists to express the number of atoms or molecules in a sample. • 1 mole = 6. 02 x 1023 “things” – 1 mole of oranges contains 6. 02 x 1023 oranges. – 1 mole of helium gas contains 6. 02 x 1023 helium atoms. – 1 mole of water contains 6. 02 x 1023 water molecules. – 1 mole of sodium chloride contains 6. 02 x 1023 sodium chloride formula units.

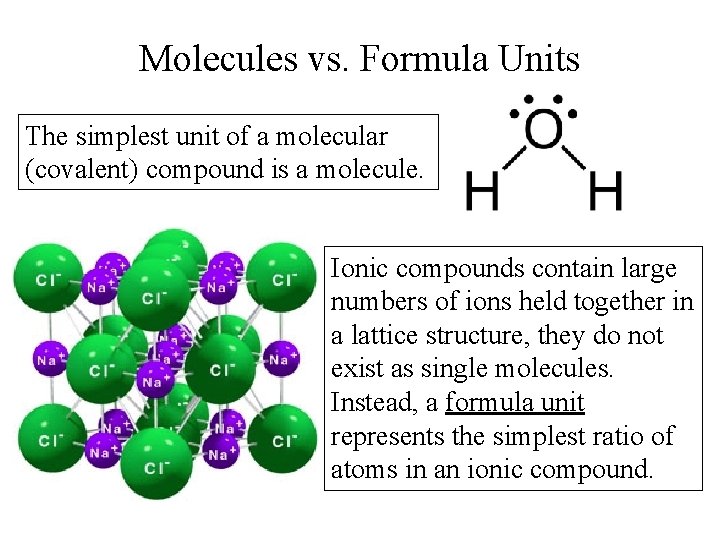
Molecules vs. Formula Units The simplest unit of a molecular (covalent) compound is a molecule. Ionic compounds contain large numbers of ions held together in a lattice structure, they do not exist as single molecules. Instead, a formula unit represents the simplest ratio of atoms in an ionic compound.

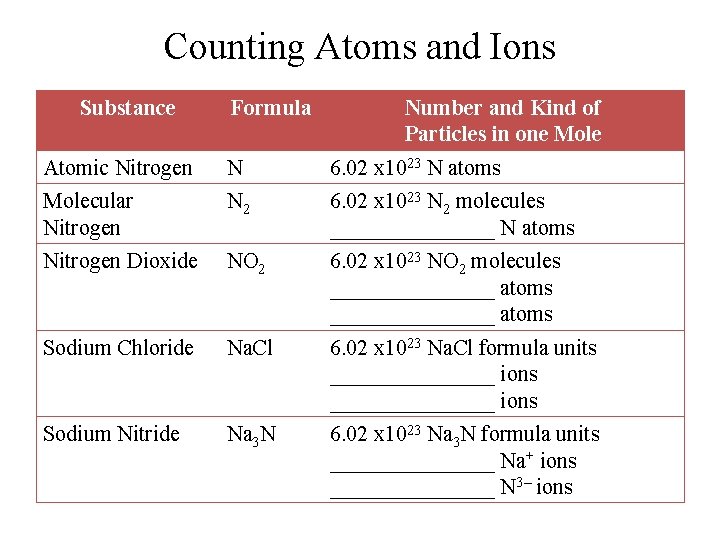
Counting Atoms and Ions Substance Formula Number and Kind of Particles in one Mole Atomic Nitrogen N 6. 02 x 1023 N atoms Molecular Nitrogen N 2 6. 02 x 1023 N 2 molecules ________ N atoms Nitrogen Dioxide NO 2 6. 02 x 1023 NO 2 molecules _______________ atoms Sodium Chloride Na. Cl 6. 02 x 1023 Na. Cl formula units _______________ ions Sodium Nitride Na 3 N 6. 02 x 1023 Na 3 N formula units ________ Na+ ions ________ N 3– ions

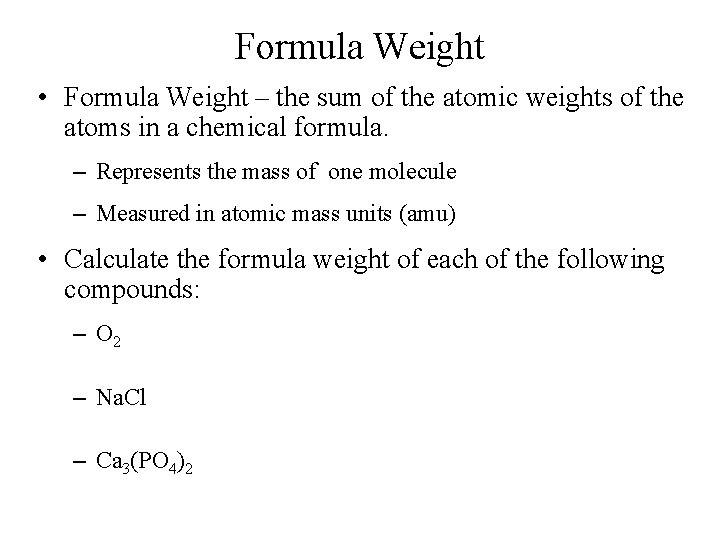
Formula Weight • Formula Weight – the sum of the atomic weights of the atoms in a chemical formula. – Represents the mass of one molecule – Measured in atomic mass units (amu) • Calculate the formula weight of each of the following compounds: – O 2 – Na. Cl – Ca 3(PO 4)2

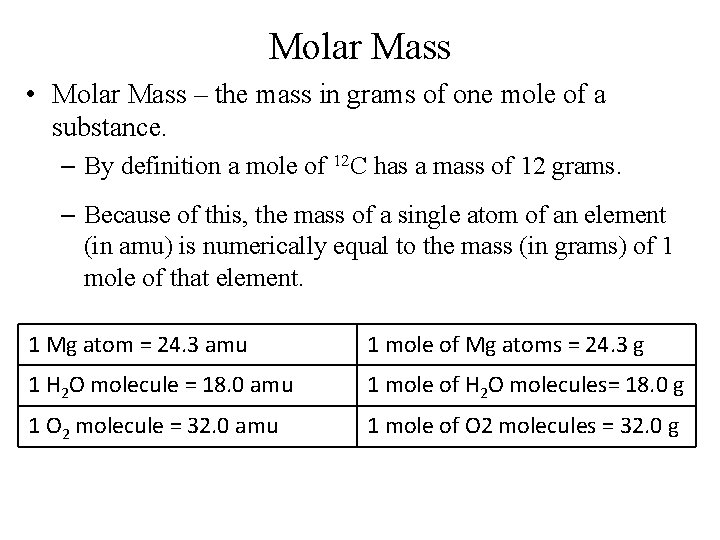
Molar Mass • Molar Mass – the mass in grams of one mole of a substance. – By definition a mole of 12 C has a mass of 12 grams. – Because of this, the mass of a single atom of an element (in amu) is numerically equal to the mass (in grams) of 1 mole of that element. 1 Mg atom = 24. 3 amu 1 mole of Mg atoms = 24. 3 g 1 H 2 O molecule = 18. 0 amu 1 mole of H 2 O molecules= 18. 0 g 1 O 2 molecule = 32. 0 amu 1 mole of O 2 molecules = 32. 0 g

Why do we need the mole? • Because atoms are extremely small and we usually deal with large amounts of them. Think about how many atoms are in 1 mole: 602, 000, 000, 000 • The mole is a useful tool for chemists because it provides a relationship between the number of atoms or molecules in a substance (which we cannot measure easily) and their mass (which we can measure easily).

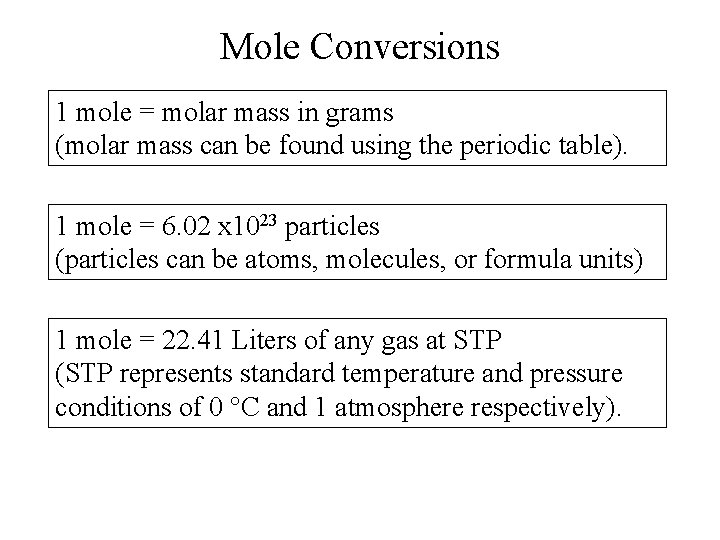
Mole Conversions 1 mole = molar mass in grams (molar mass can be found using the periodic table). 1 mole = 6. 02 x 1023 particles (particles can be atoms, molecules, or formula units) 1 mole = 22. 41 Liters of any gas at STP (STP represents standard temperature and pressure conditions of 0 °C and 1 atmosphere respectively).