Unit 3 Energy is Conserved and its Transformation


















- Slides: 49

Unit 3 - Energy is Conserved and its Transformation Affects Living Things and the Environment Section 3. 2 How is Energy Transformed?


Concept #1 - Energy is Transformed in Chemical Reactions (p 222) • How is chemical potential energy transformed to run a car or contract a muscle? Energy Transformation in Chemical Reactions (p 222) • Chemical reactions can transform some of the energy stored in chemical bonds into other forms of energy. The materials involved in a chemical reaction are described as a system.

• During a chemical reaction, some energy is released or absorbed from the surroundings. Usually, this form of energy is thermal (heat), but other forms such as light or sound can be released as well. • The chemical bonds in the reactants and products determines the amount of energy that will be transformed. The amount of energy stored in the bonds varies in different compounds. • Some compounds would release little energy if formed from elements. • Some compounds would release a lot of energy if formed from elements.

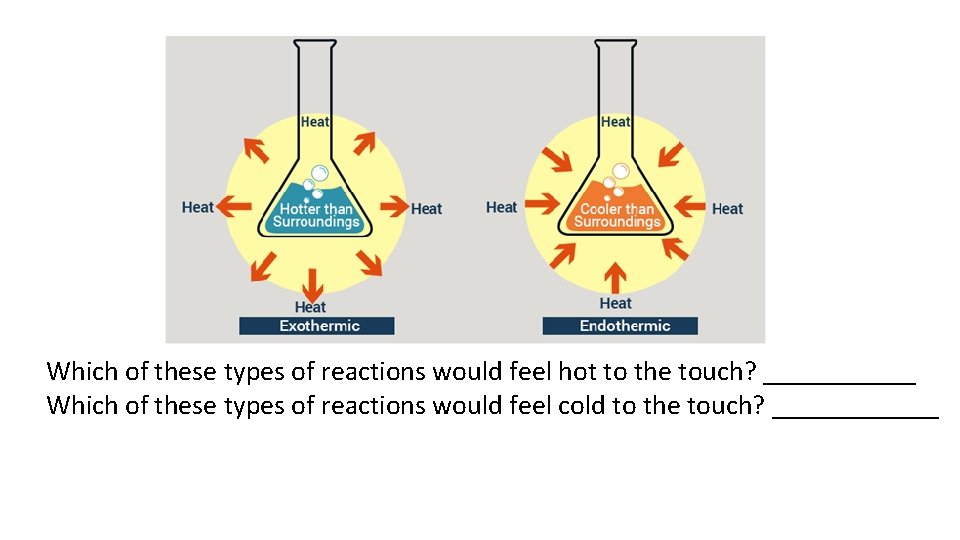
• A chemical reaction may release or absorb energy. We describe each of these scenarios as: • Exothermic: If the reactants are higher in chemical potential energy than the products, energy is released by the system during the reaction • Endothermic: If the reactants are lower in chemical potential energy than the products, energy is absorbed from the surroundings during the reaction.

Which of these types of reactions would feel hot to the touch? ______ Which of these types of reactions would feel cold to the touch? ______

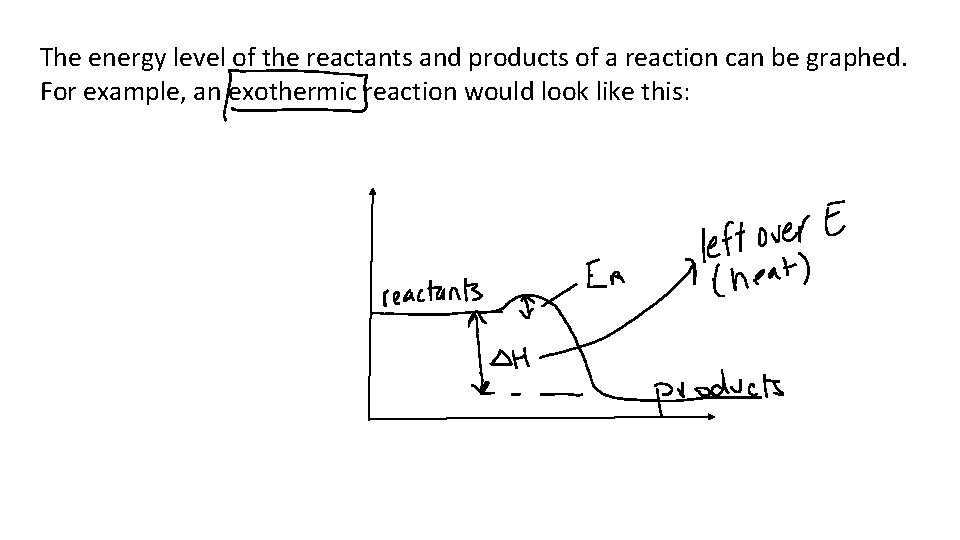
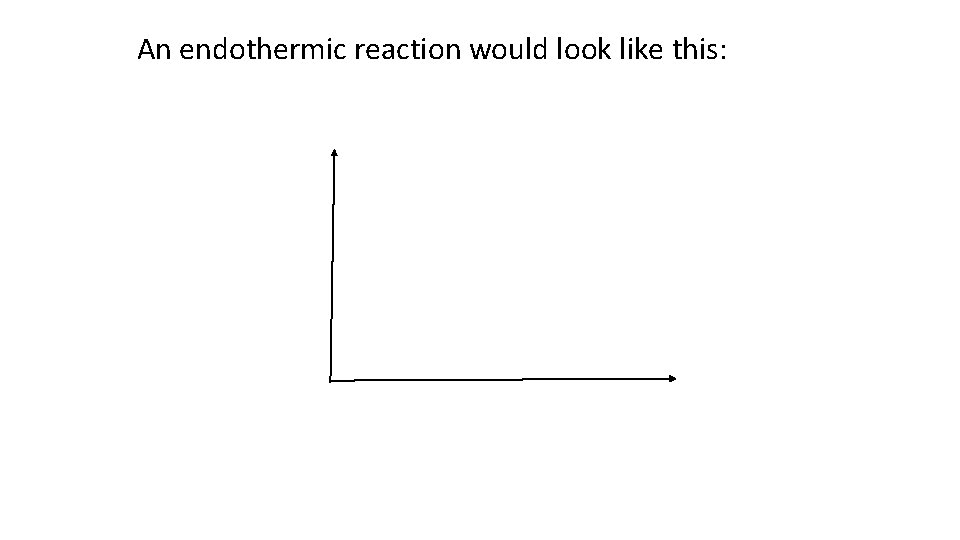
The energy level of the reactants and products of a reaction can be graphed. For example, an exothermic reaction would look like this:

An endothermic reaction would look like this:

Chemical Reactions in Animals and Plants (p 224) 1. Cellular Respiration All living things need energy to live. In most organisms, this is done by the process called cellular respiration. This is a series of chemical reactions in which glucose and oxygen react to form carbon dioxide, water and energy. Both animals AND plants have mitochondria and use cellular respiration.

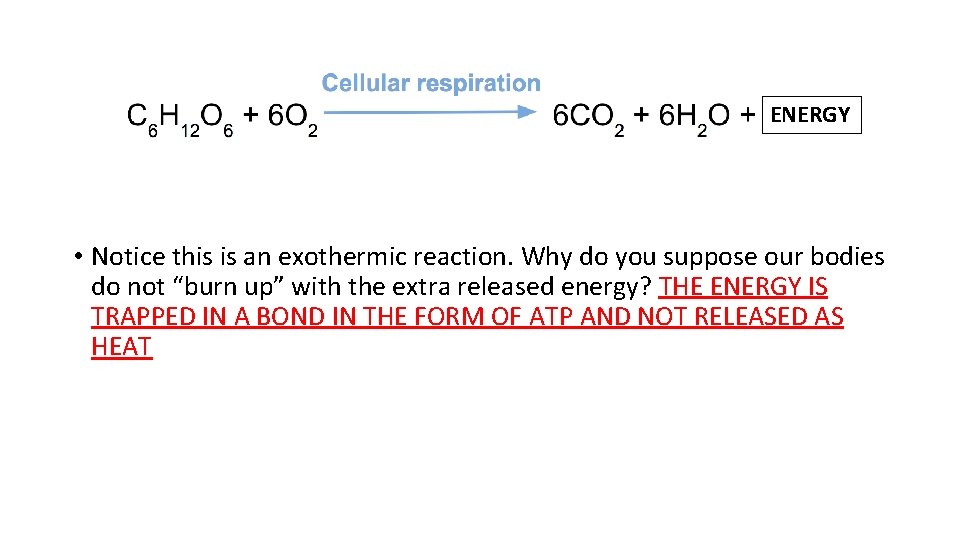
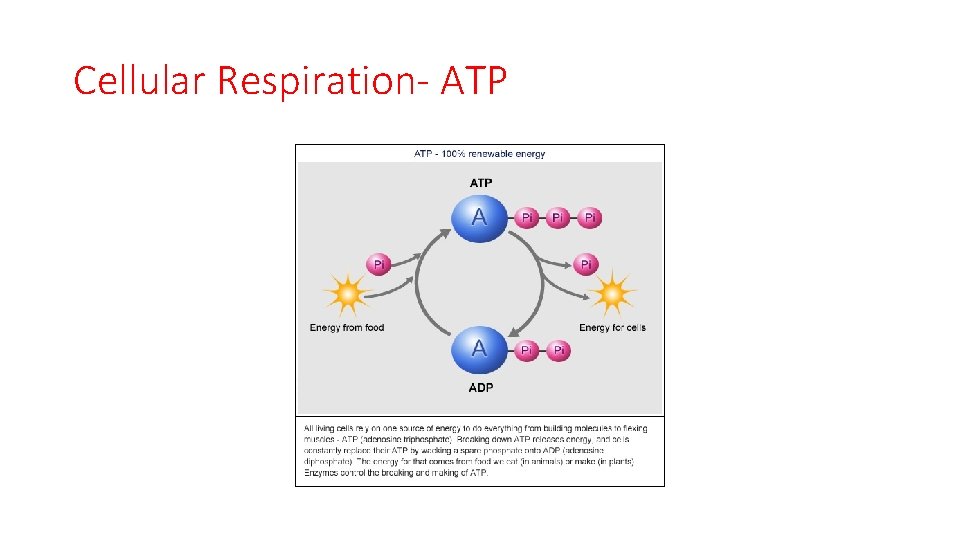
ENERGY • Notice this is an exothermic reaction. Why do you suppose our bodies do not “burn up” with the extra released energy? THE ENERGY IS TRAPPED IN A BOND IN THE FORM OF ATP AND NOT RELEASED AS HEAT

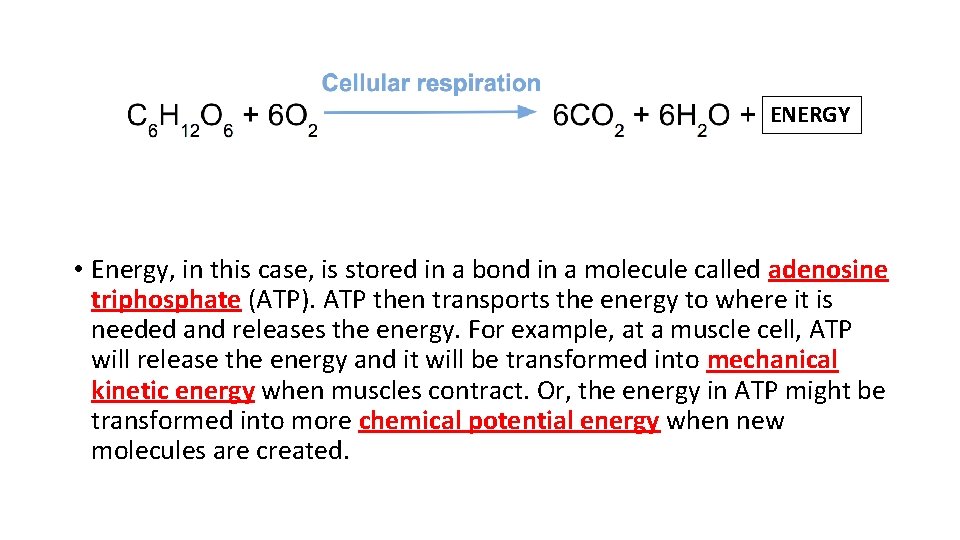
ENERGY • Energy, in this case, is stored in a bond in a molecule called adenosine triphosphate (ATP). ATP then transports the energy to where it is needed and releases the energy. For example, at a muscle cell, ATP will release the energy and it will be transformed into mechanical kinetic energy when muscles contract. Or, the energy in ATP might be transformed into more chemical potential energy when new molecules are created.

Cellular Respiration- ATP



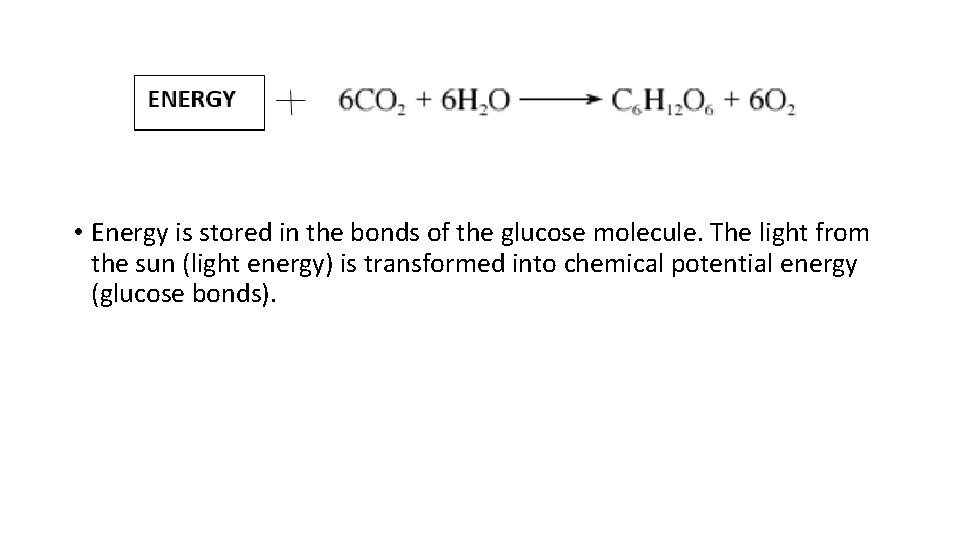
2. Photosynthesis • Where does glucose come from? ______ • Photosynthesis is a chemical reaction where carbon dioxide and water combine with the addition of light energy to produce glucose and oxygen. • This process occurs mainly in plants, some algae and some other microscopic organisms.

• Energy is stored in the bonds of the glucose molecule. The light from the sun (light energy) is transformed into chemical potential energy (glucose bonds).


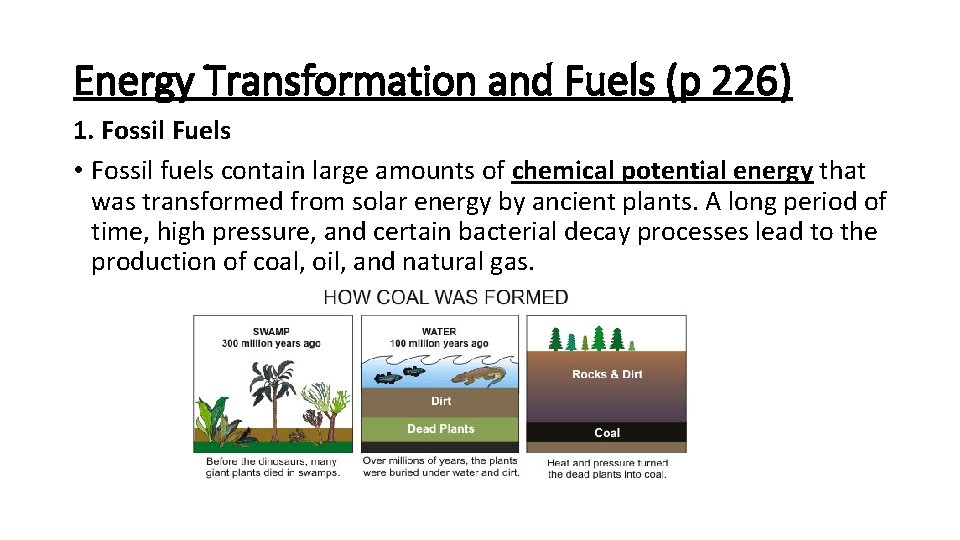
Energy Transformation and Fuels (p 226) 1. Fossil Fuels • Fossil fuels contain large amounts of chemical potential energy that was transformed from solar energy by ancient plants. A long period of time, high pressure, and certain bacterial decay processes lead to the production of coal, oil, and natural gas.

2. Fossil Fuel Combustion • The burning of fossil fuels releases tonnes of carbon dioxide into the air, which contributes to global warming. This is a combustion reaction. The combustion of oil, for example, would be: 2 C 8 H 18 (l) + 25 O 2(g) 16 CO 2(g) + 18 H 2 O(g) + energy **circle the carbon dioxide in the above equation. What is the energy used for? _____
3. Fuel Cells • Other forms of energy emit fewer pollutants. Fuel cells, for example, use hydrogen and oxygen to transform chemical potential energy into electrical energy. The only product for this reaction is water. 2 H 2(g) + O 2(g) 2 H 2 O(g) + energy



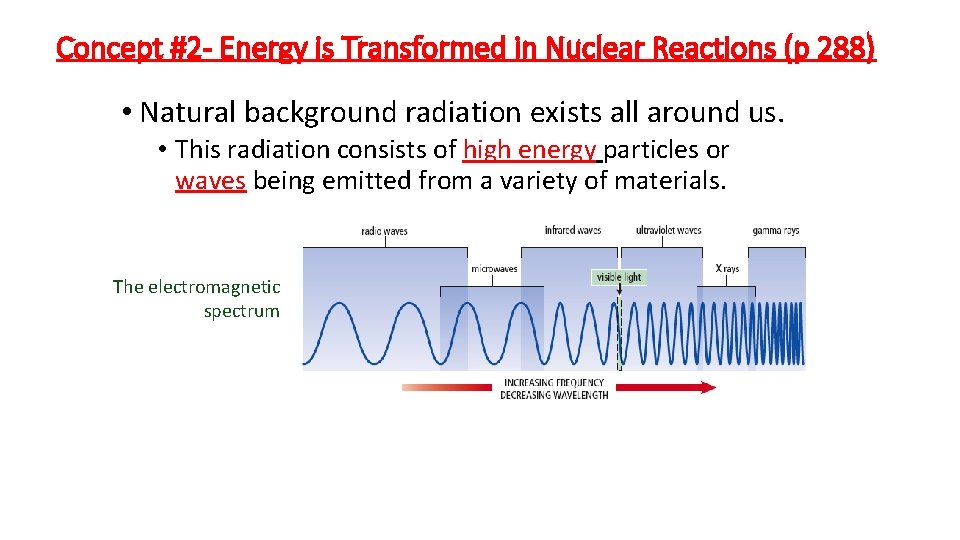
Concept #2 - Energy is Transformed in Nuclear Reactions (p 288) • Natural background radiation exists all around us. • This radiation consists of high energy particles or waves being emitted from a variety of materials. The electromagnetic spectrum

• Radioactivity is the release of high-energy particles or waves. • Being exposed to radioactive materials can be beneficial or harmful. • X rays, radiation therapy, and electricity generation are beneficial. • High-energy particles and waves damage DNA in our cells. • Video: What does harmful radiation do to the body? https: //www. youtube. com/watch? v=hh. Uu. FCr. DOlw

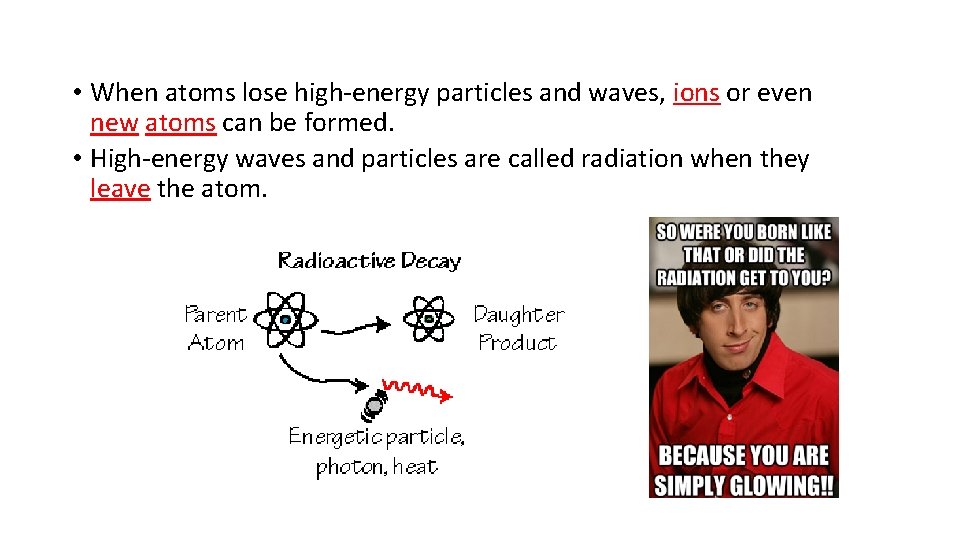
• When atoms lose high-energy particles and waves, ions or even new atoms can be formed. • High-energy waves and particles are called radiation when they leave the atom.

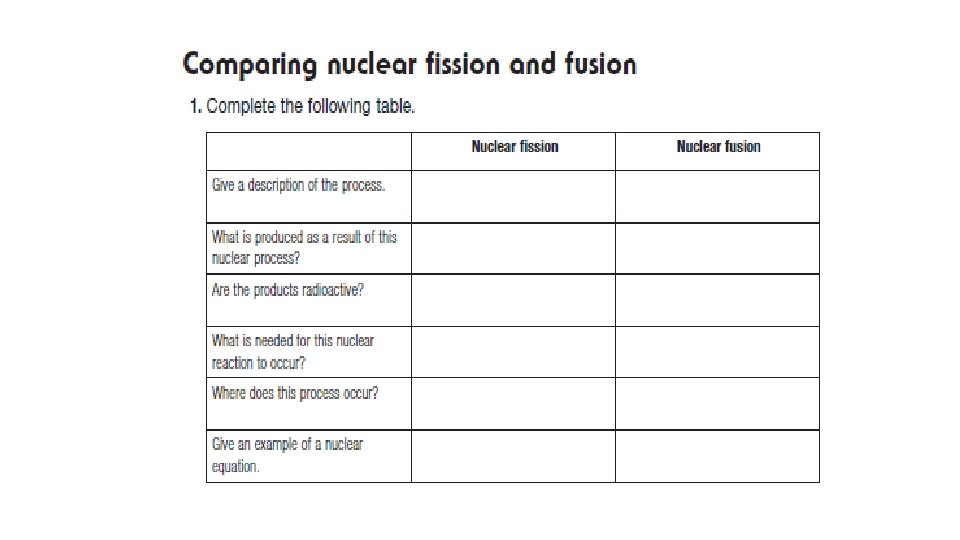
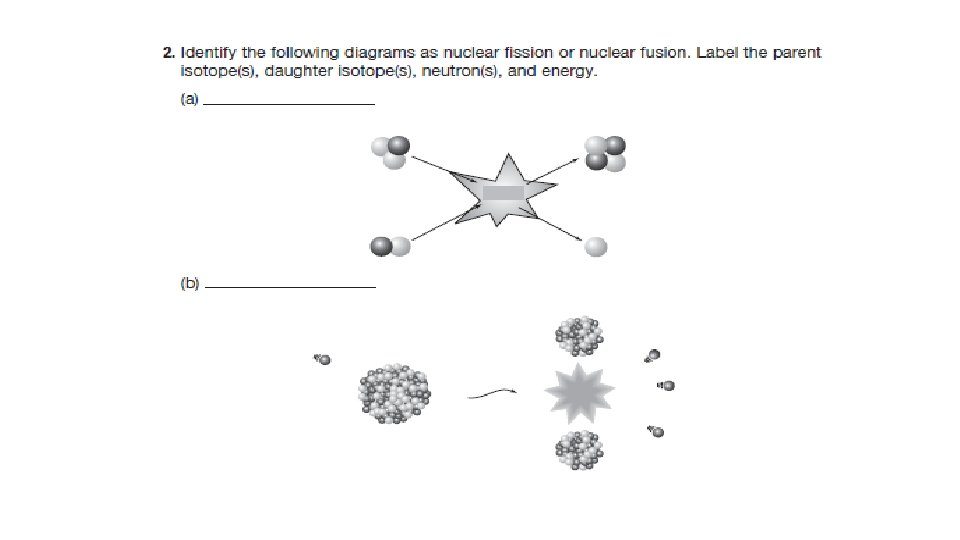
Nuclear Reactions (p. 231) • Nuclear fission and fusion are processes that involve extremely large amounts of energy. • Fission = the splitting of nuclei • Fusion = the joining of nuclei

Nuclear Fission • Nuclear energy used to produce power comes from fission. • Nuclear fission is the splitting of one heavy nucleus into two or more smaller nuclei, some sub-atomic particles, and energy. • A heavy nucleus is usually unstable, due to many positive protons pushing apart. • When fission occurs: 1. Energy is produced. 2. Neutrons are released. Albert Einstein’s famous equation E = mc 2 illustrates the energy found in even small amounts of matter

Nuclear Fission • Nuclear reactions are different than chemical reactions. • In chemical reactions, mass is conserved, and energy changes are relatively small. • There are no changes to the nuclei in chemical reactions. • In nuclear reactions, the actual nucleus of atoms changes. • Protons, neutrons, electrons, and/or gamma rays can be lost or gained. • Small changes of mass = huge changes in energy

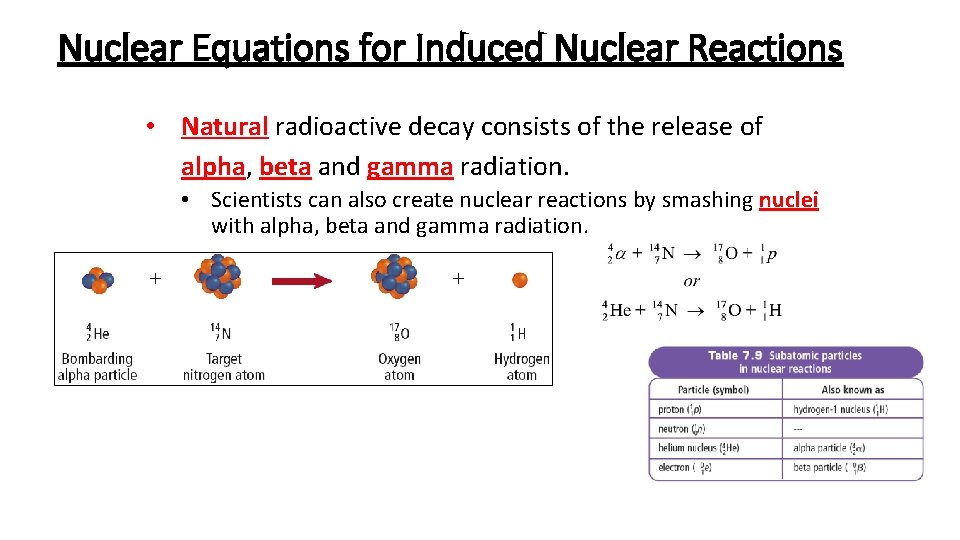
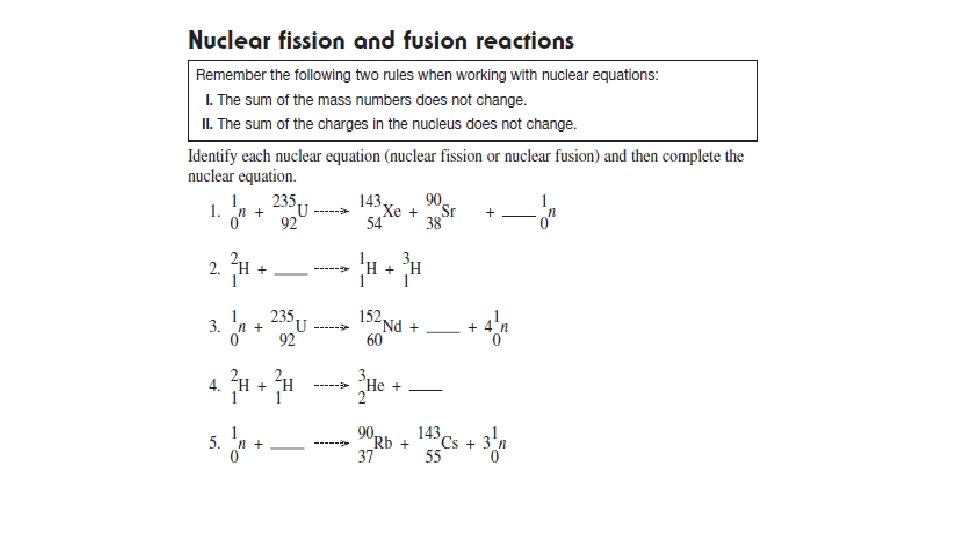
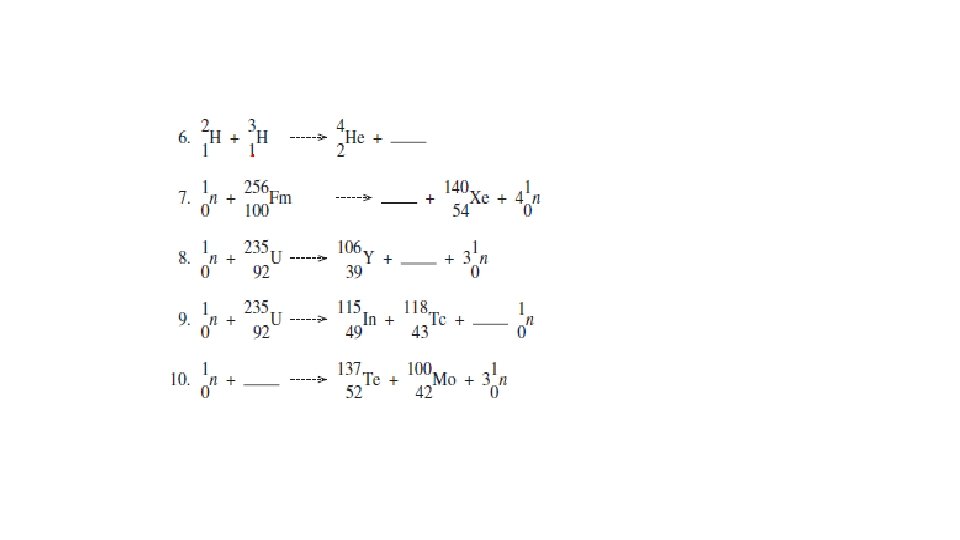
Nuclear Equations for Induced Nuclear Reactions • Natural radioactive decay consists of the release of alpha, beta and gamma radiation. • Scientists can also create nuclear reactions by smashing nuclei with alpha, beta and gamma radiation.

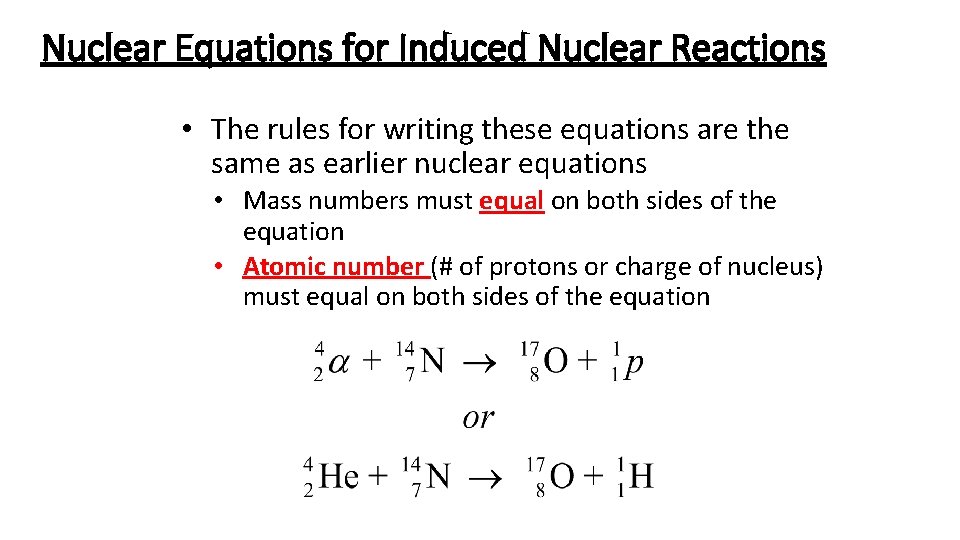
Nuclear Equations for Induced Nuclear Reactions • The rules for writing these equations are the same as earlier nuclear equations • Mass numbers must equal on both sides of the equation • Atomic number (# of protons or charge of nucleus) must equal on both sides of the equation

Nuclear Fission of Uranium-235 • It is much easier to crash a neutral neutron than a positive proton into a nucleus to release energy. • Most nuclear fission reactors and weapons use this principle. • A neutron, , crashes into an atom of stable uranium-235 to create unstable uranium-236, which then undergoes radioactive decay. • After several steps, atoms of krypton and barium are formed, along with the release of three neutrons and huge quantities of energy. The induced nuclear fission of uranium-235. This nuclear reaction is the origin of nuclear power and nuclear bombs.

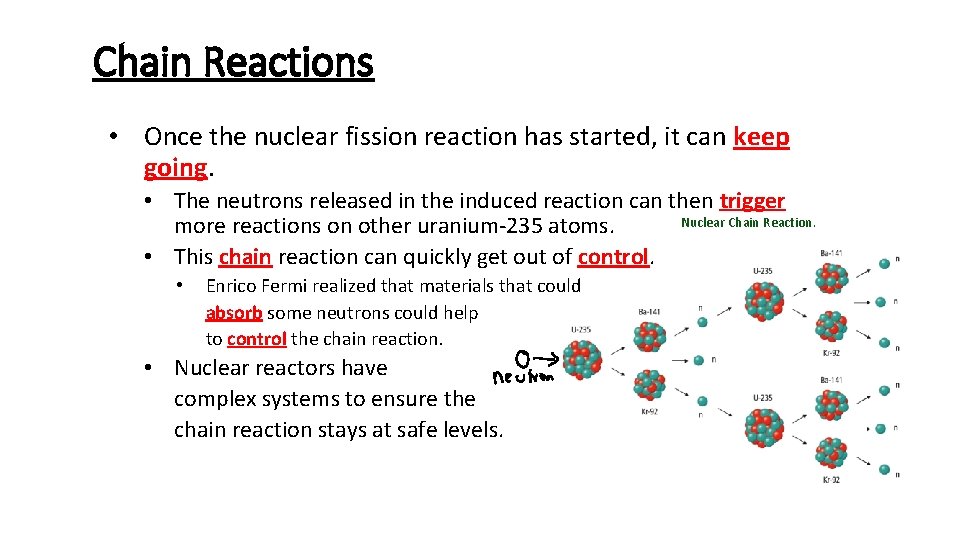
Chain Reactions • Once the nuclear fission reaction has started, it can keep going. • The neutrons released in the induced reaction can then trigger Nuclear Chain Reaction. more reactions on other uranium-235 atoms. • This chain reaction can quickly get out of control. • Enrico Fermi realized that materials that could absorb some neutrons could help to control the chain reaction. • Nuclear reactors have complex systems to ensure the chain reaction stays at safe levels.

Chain Reactions • An uncontrolled chain reaction can result in a violent nuclear explosion. To control the reaction, control rods are used to absorb neutrons to slow the reaction. • The spent fuel rods and control rods are biohazardous and must be stored properly • Nuclear bombs are created using this concept.

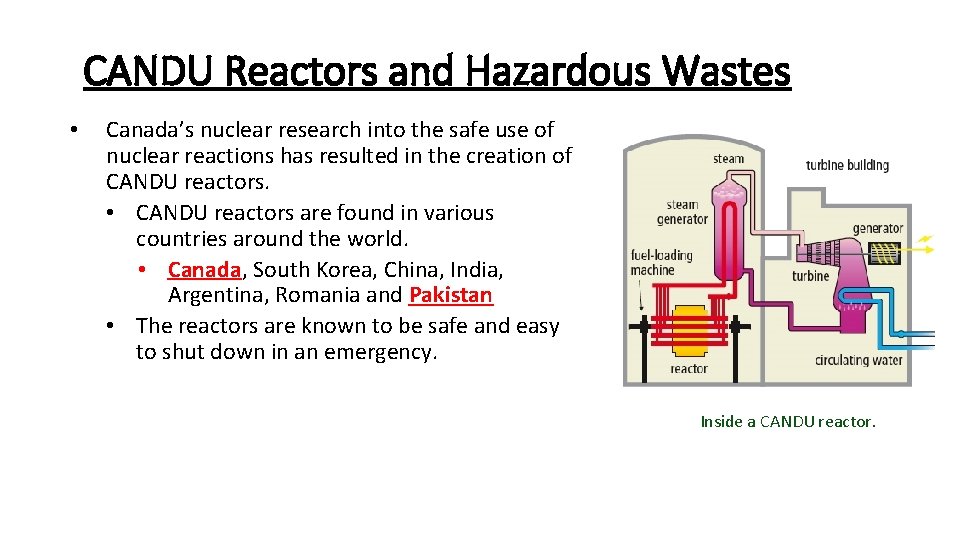
CANDU Reactors and Hazardous Wastes • Canada’s nuclear research into the safe use of nuclear reactions has resulted in the creation of CANDU reactors. • CANDU reactors are found in various countries around the world. • Canada, South Korea, China, India, Argentina, Romania and Pakistan • The reactors are known to be safe and easy to shut down in an emergency. Inside a CANDU reactor.


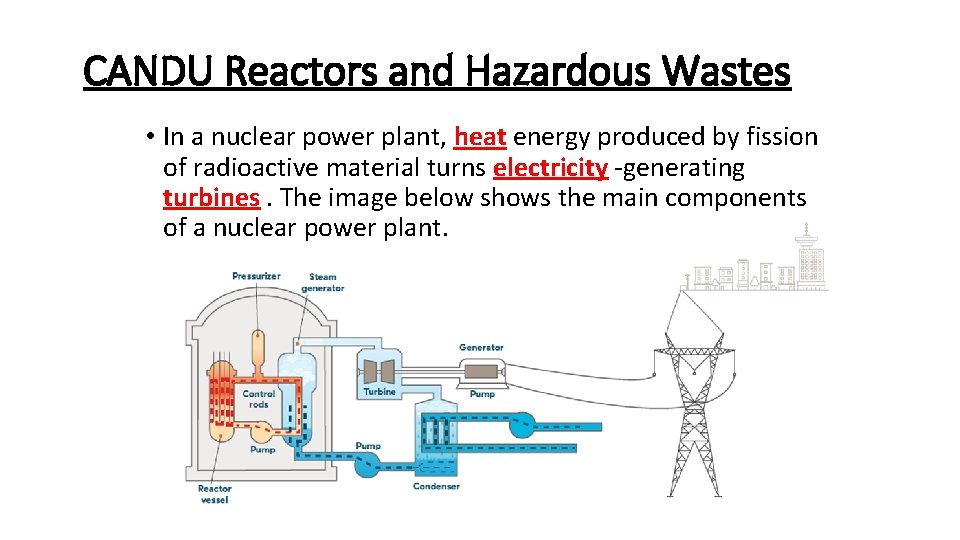
CANDU Reactors and Hazardous Wastes • In a nuclear power plant, heat energy produced by fission of radioactive material turns electricity -generating turbines. The image below shows the main components of a nuclear power plant.

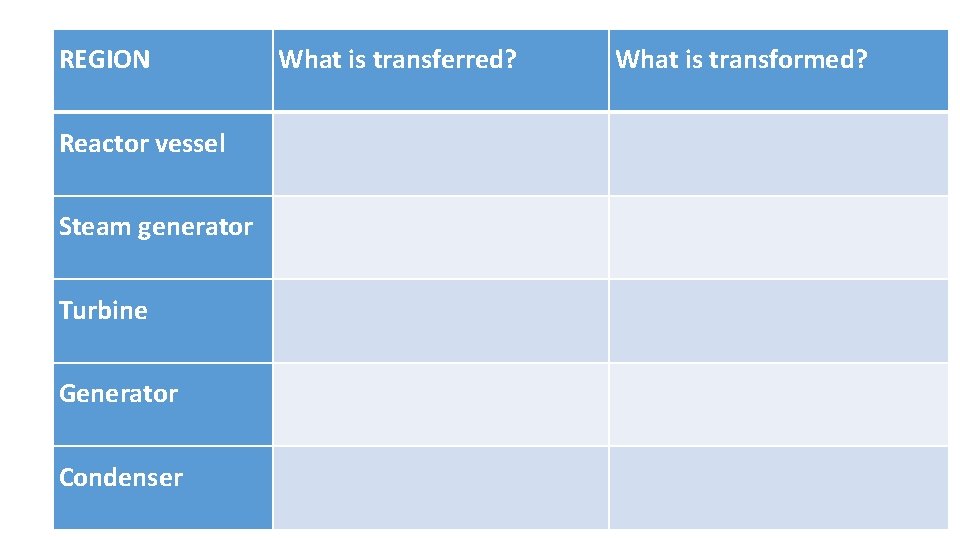
REGION What is transferred? What is transformed? Reactor vessel Steam generator Turbine Generator Condenser

• Hazardous wastes produced by nuclear reactions are problematic. • Some products are very radioactive, and must be stored away from living things. • Most of this waste is buried underground or stored in concrete.

Radioactive Waste Management- Bury It! http: //www. world-nuclear. org/information-library/nuclear-fuel -cycle/nuclear-wastes/radioactive-waste-management. aspx

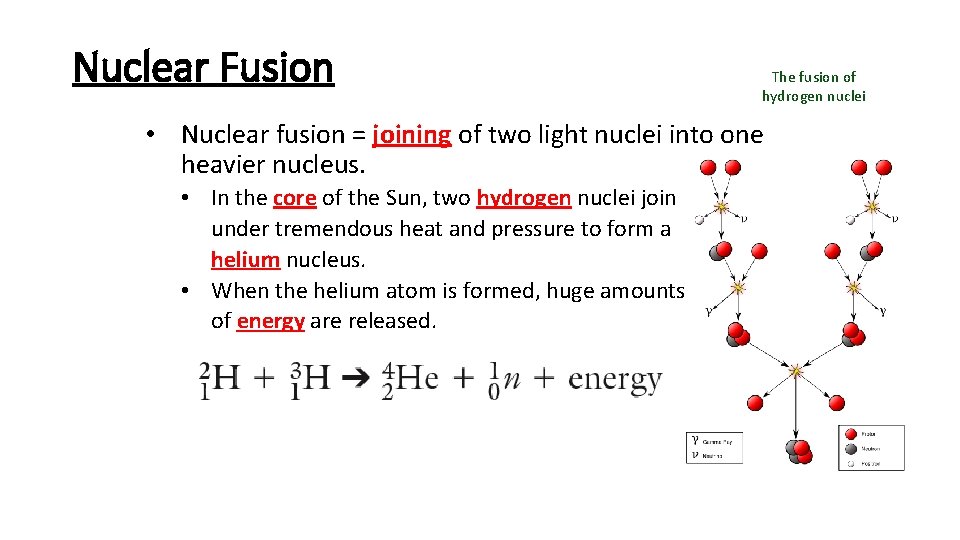
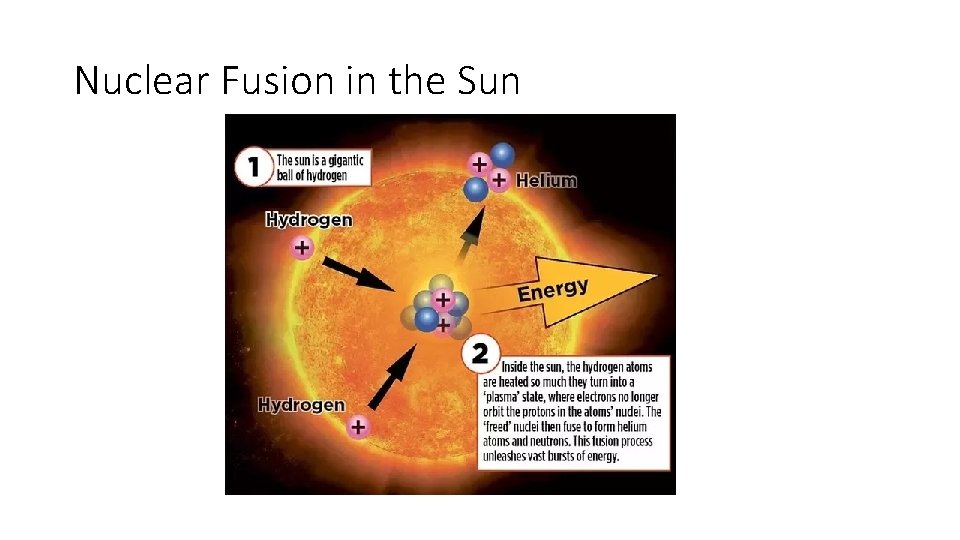
Nuclear Fusion The fusion of hydrogen nuclei • Nuclear fusion = joining of two light nuclei into one heavier nucleus. • In the core of the Sun, two hydrogen nuclei join under tremendous heat and pressure to form a helium nucleus. • When the helium atom is formed, huge amounts of energy are released.

Nuclear Fusion in the Sun

Nuclear Fusion • Scientists cannot yet find a safe, manageable method to harness the energy of nuclear fusion. • So-called “cold fusion” would occur at temperatures and pressures that could be controlled.





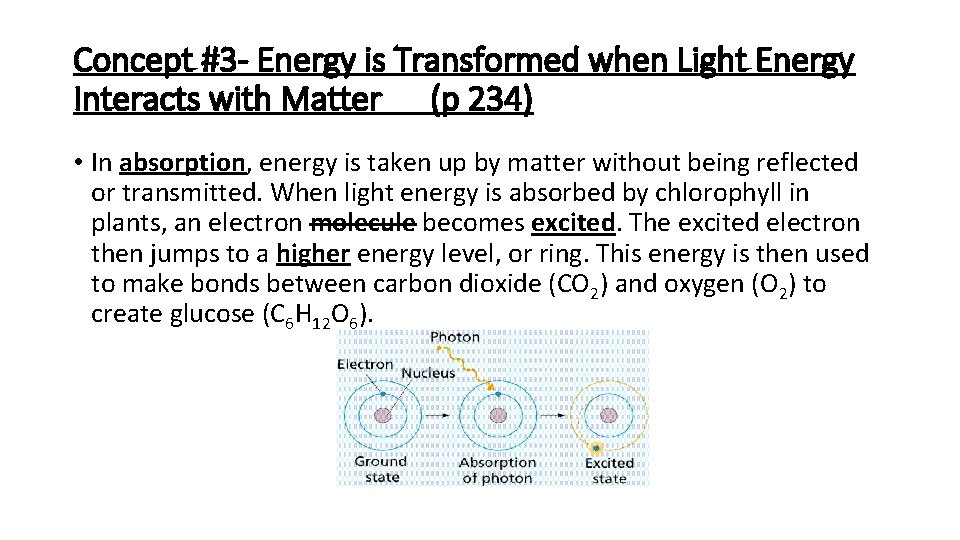
Concept #3 - Energy is Transformed when Light Energy Interacts with Matter (p 234) • In absorption, energy is taken up by matter without being reflected or transmitted. When light energy is absorbed by chlorophyll in plants, an electron molecule becomes excited. The excited electron then jumps to a higher energy level, or ring. This energy is then used to make bonds between carbon dioxide (CO 2) and oxygen (O 2) to create glucose (C 6 H 12 O 6).

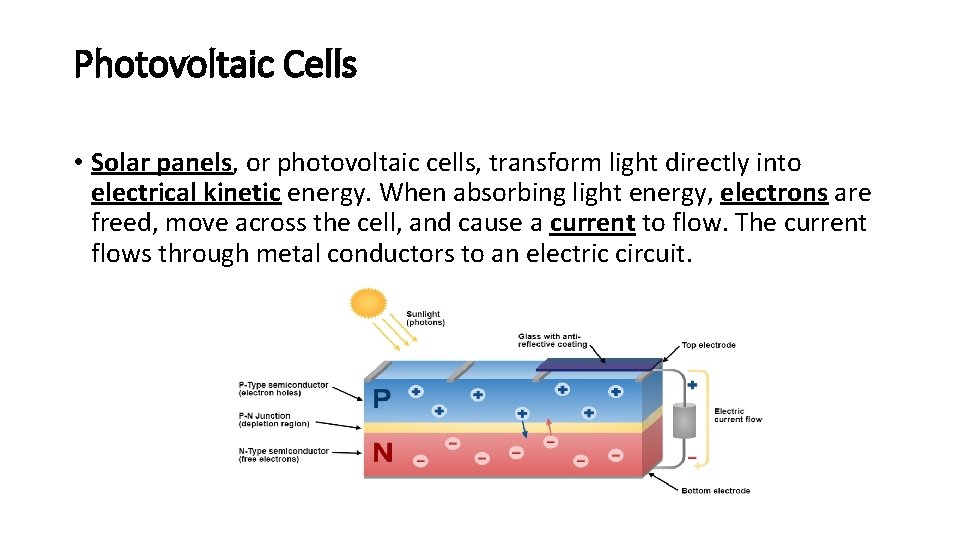
Photovoltaic Cells • Solar panels, or photovoltaic cells, transform light directly into electrical kinetic energy. When absorbing light energy, electrons are freed, move across the cell, and cause a current to flow. The current flows through metal conductors to an electric circuit.

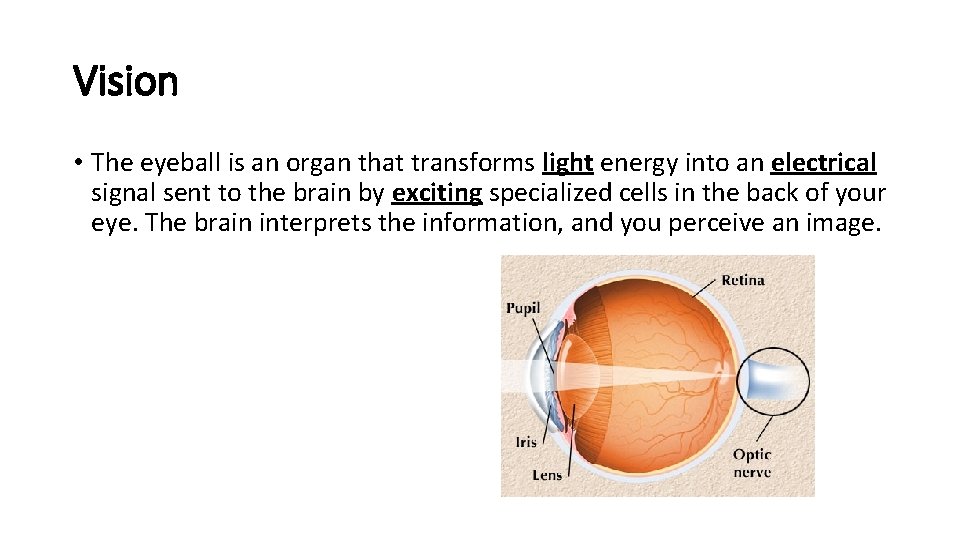
Vision • The eyeball is an organ that transforms light energy into an electrical signal sent to the brain by exciting specialized cells in the back of your eye. The brain interprets the information, and you perceive an image.