Types of Reactions 5 types of reactions 1
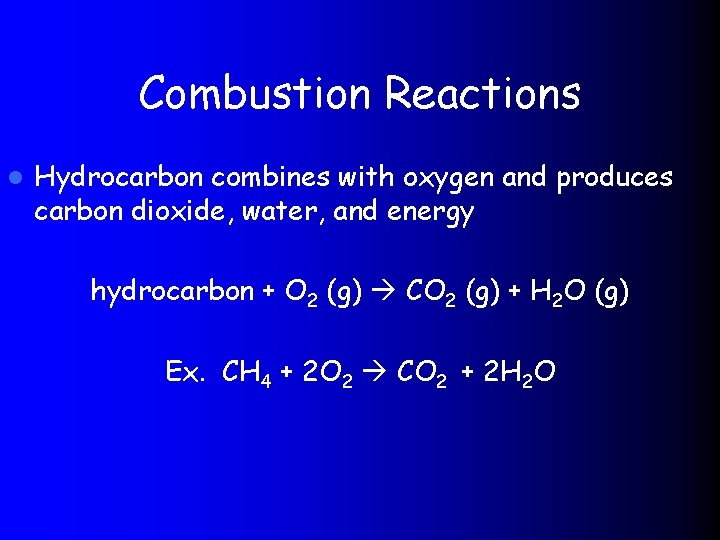
- Slides: 8

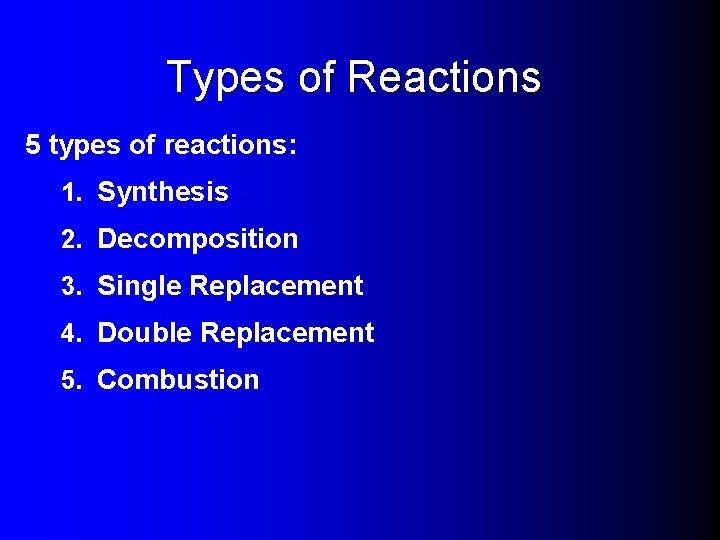
Types of Reactions 5 types of reactions: 1. Synthesis 2. Decomposition 3. Single Replacement 4. Double Replacement 5. Combustion

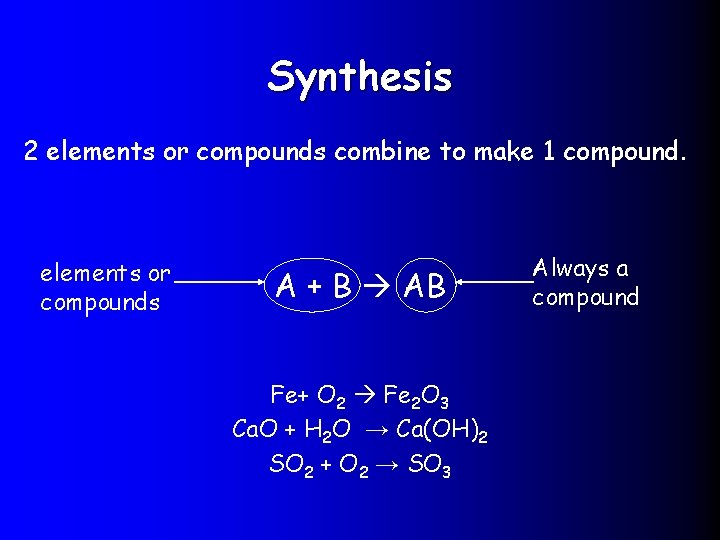
Synthesis 2 elements or compounds combine to make 1 compound. elements or compounds A + B AB Fe+ O 2 Fe 2 O 3 Ca. O + H 2 O → Ca(OH)2 SO 2 + O 2 → SO 3 Always a compound

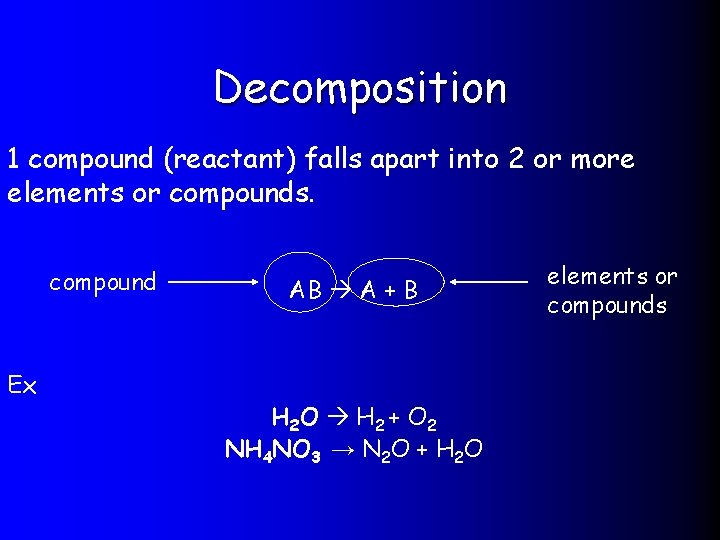
Decomposition 1 compound (reactant) falls apart into 2 or more elements or compounds. compound Ex AB A + B H 2 O H 2 + O 2 NH 4 NO 3 → N 2 O + H 2 O elements or compounds

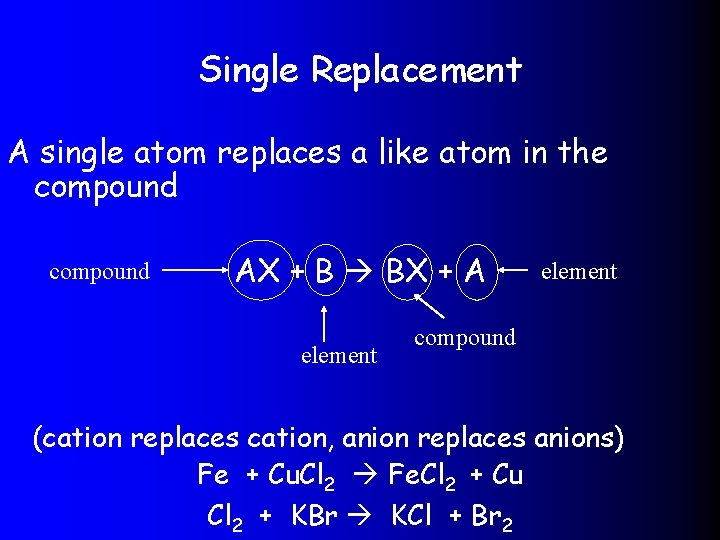
Single Replacement A single atom replaces a like atom in the compound AX + B BX + A element compound (cation replaces cation, anion replaces anions) Fe + Cu. Cl 2 Fe. Cl 2 + Cu Cl 2 + KBr KCl + Br 2

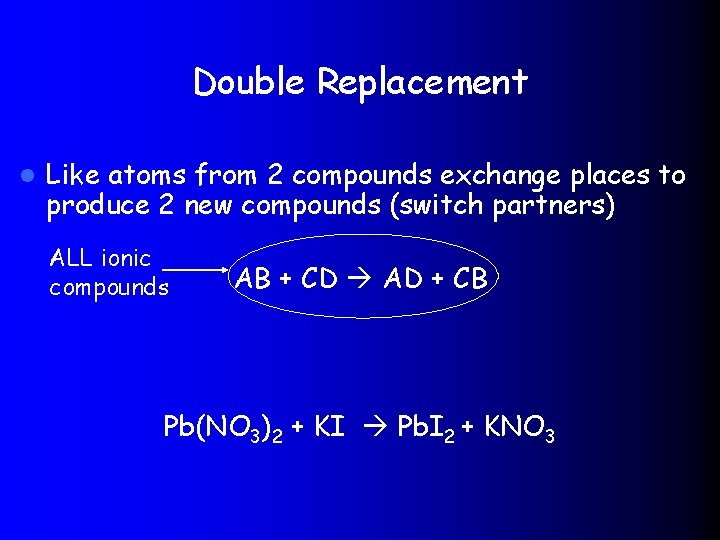
Double Replacement l Like atoms from 2 compounds exchange places to produce 2 new compounds (switch partners) ALL ionic compounds AB + CD AD + CB Pb(NO 3)2 + KI Pb. I 2 + KNO 3
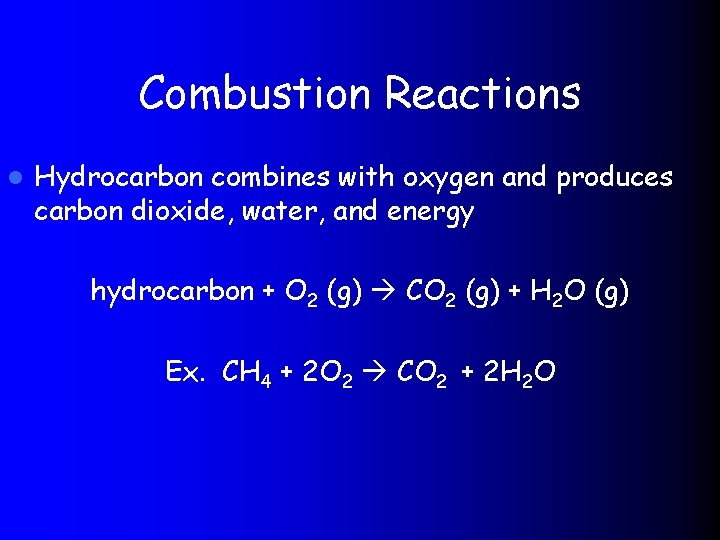
Combustion Reactions l Hydrocarbon combines with oxygen and produces carbon dioxide, water, and energy hydrocarbon + O 2 (g) CO 2 (g) + H 2 O (g) Ex. CH 4 + 2 O 2 CO 2 + 2 H 2 O

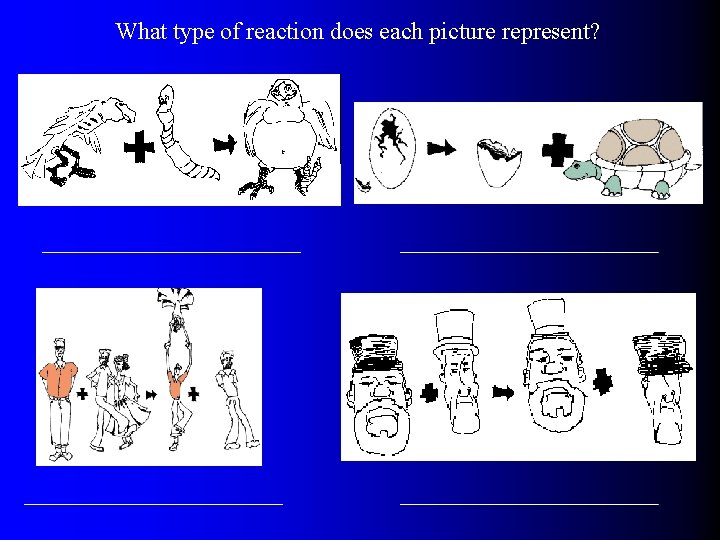
What type of reaction does each picture represent?

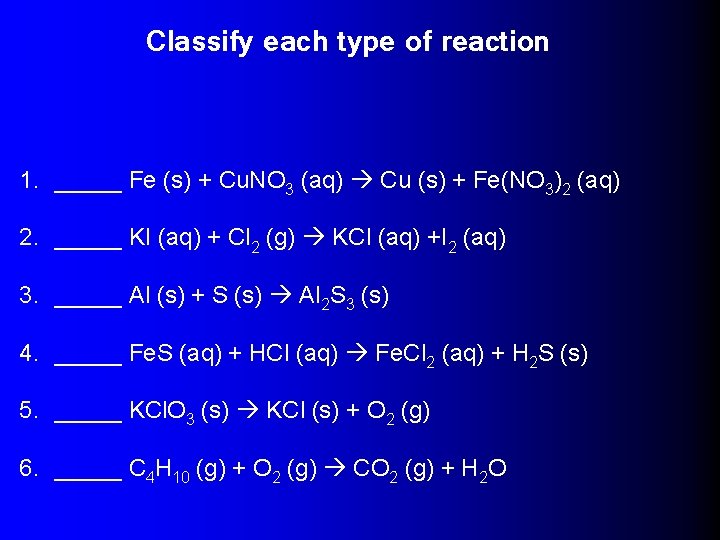
Classify each type of reaction 1. _____ Fe (s) + Cu. NO 3 (aq) Cu (s) + Fe(NO 3)2 (aq) 2. _____ KI (aq) + Cl 2 (g) KCl (aq) +I 2 (aq) 3. _____ Al (s) + S (s) Al 2 S 3 (s) 4. _____ Fe. S (aq) + HCl (aq) Fe. Cl 2 (aq) + H 2 S (s) 5. _____ KCl. O 3 (s) KCl (s) + O 2 (g) 6. _____ C 4 H 10 (g) + O 2 (g) CO 2 (g) + H 2 O
 Section 2 reinforcement classifying chemical reactions
Section 2 reinforcement classifying chemical reactions Balancing redox reactions in acidic solution
Balancing redox reactions in acidic solution Section 2 reinforcement classifying chemical reactions
Section 2 reinforcement classifying chemical reactions Chemical reactions section 3 reactions in aqueous solutions
Chemical reactions section 3 reactions in aqueous solutions Unit 5 chemical reactions answers
Unit 5 chemical reactions answers Types of chemical reactions redox
Types of chemical reactions redox Types of reaction
Types of reaction Types of reactions
Types of reactions Single replacement reaction examples
Single replacement reaction examples















