TwoYear Outcomes After Everolimus or Sirolimus Eluting Stents


![All Cause Death 10 RR 0. 93 [95% CI, 0. 61 -1. 43]; P=0. All Cause Death 10 RR 0. 93 [95% CI, 0. 61 -1. 43]; P=0.](https://slidetodoc.com/presentation_image_h/96cc846388df962c5e9ea0a5fdbfaf91/image-14.jpg)
![Target Lesion Revascularization 100 RR 0. 73 [95% CI, 0. 52 -1. 01], P=0. Target Lesion Revascularization 100 RR 0. 73 [95% CI, 0. 52 -1. 01], P=0.](https://slidetodoc.com/presentation_image_h/96cc846388df962c5e9ea0a5fdbfaf91/image-17.jpg)

- Slides: 23

Two-Year Outcomes After Everolimus- or Sirolimus. Eluting Stents in Patients With Coronary Artery Disease in the ISAR-TEST 4 Trial Robert A. Byrne, Adnan Kastrati, Klaus Tiroch, Steffen Massberg, Anna Wieczorek, Karl-Ludwig Laugwitz, Stefanie Schulz, Jürgen Pache, Massimiliano Fusaro, Melchior Seyfarth, Albert Schömig, Julinda Mehilli Deutsches Herzzentrum & 1. Medizinische Klinik, Klinikum rechts der Isar, Technische Universität, Munich. Germany

Presenter Disclosure Information: Nothing to disclose

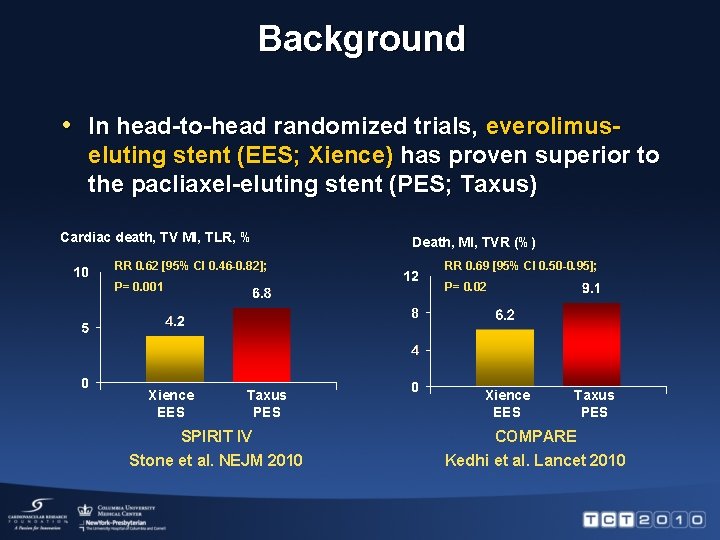
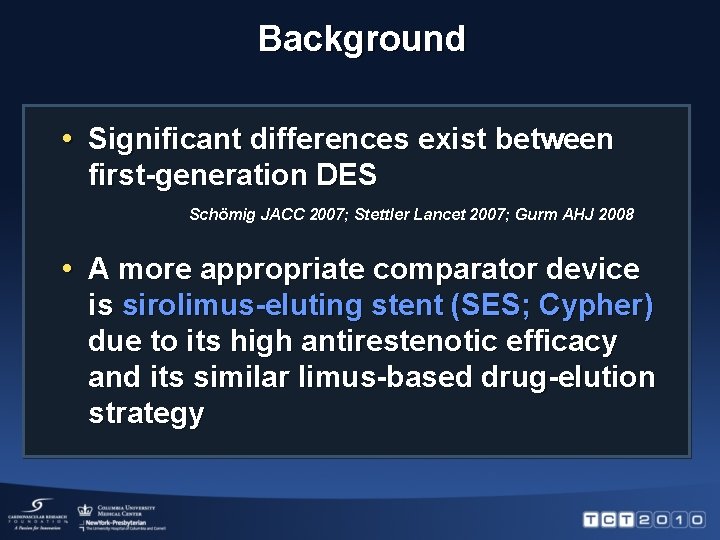
Background • In head-to-head randomized trials, everolimuseluting stent (EES; Xience) has proven superior to the pacliaxel-eluting stent (PES; Taxus) Cardiac death, TV MI, TLR, % Death, MI, TVR (%) RR 0. 62 [95% CI 0. 46 -0. 82]; RR 0. 69 [95% CI 0. 50 -0. 95]; P= 0. 001 P= 0. 02 Xience EES Taxus PES SPIRIT IV Stone et al. NEJM 2010 Xience EES Taxus PES COMPARE Kedhi et al. Lancet 2010

Background • Significant differences exist between first-generation DES Schömig JACC 2007; Stettler Lancet 2007; Gurm AHJ 2008 • A more appropriate comparator device is sirolimus-eluting stent (SES; Cypher) due to its high antirestenotic efficacy and its similar limus-based drug-elution strategy

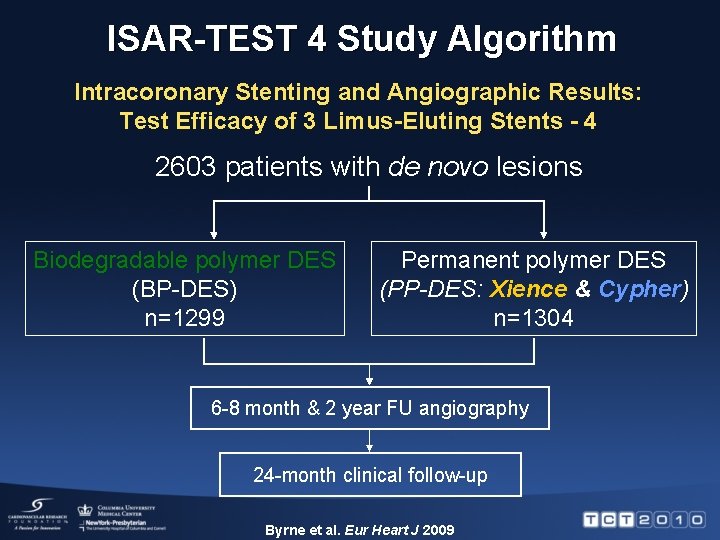
ISAR-TEST 4 Study Algorithm Intracoronary Stenting and Angiographic Results: Test Efficacy of 3 Limus-Eluting Stents - 4 2603 patients with de novo lesions Biodegradable polymer DES (BP-DES) n=1299 Permanent polymer DES (PP-DES: Xience & Cypher) n=1304 6 -8 month & 2 year FU angiography 24 -month clinical follow-up Byrne et al. Eur Heart J 2009

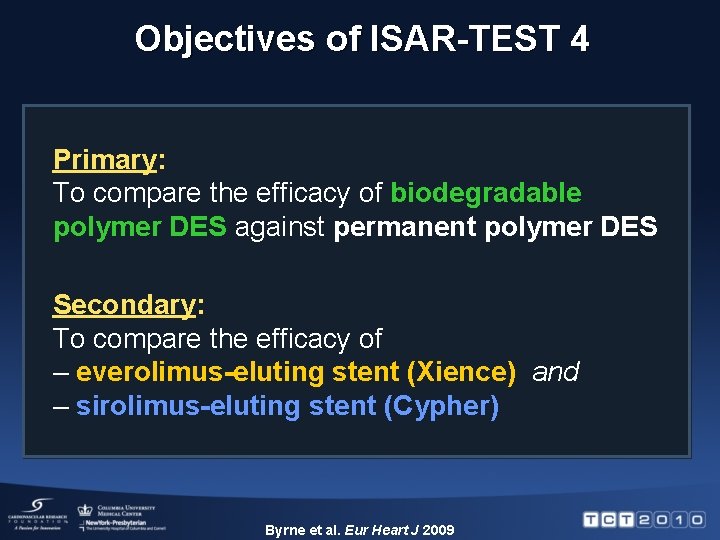
Objectives of ISAR-TEST 4 Primary: To compare the efficacy of biodegradable polymer DES against permanent polymer DES Secondary: To compare the efficacy of – everolimus-eluting stent (Xience) and – sirolimus-eluting stent (Cypher) Byrne et al. Eur Heart J 2009

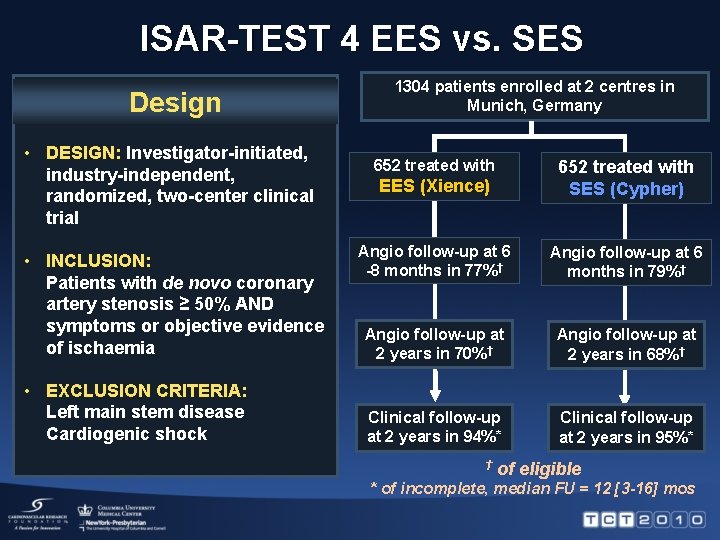
ISAR-TEST 4 EES vs. SES Design • DESIGN: Investigator-initiated, industry-independent, randomized, two-center clinical trial • INCLUSION: Patients with de novo coronary artery stenosis ≥ 50% AND symptoms or objective evidence of ischaemia • EXCLUSION CRITERIA: Left main stem disease Cardiogenic shock 1304 patients enrolled at 2 centres in Munich, Germany 652 treated with EES (Xience) 652 treated with SES (Cypher) Angio follow-up at 6 -8 months in 77%† Angio follow-up at 6 months in 79%† Angio follow-up at 2 years in 70%† Angio follow-up at 2 years in 68%† Clinical follow-up at 2 years in 94%* Clinical follow-up at 2 years in 95%* † of eligible * of incomplete, median FU = 12 [3 -16] mos

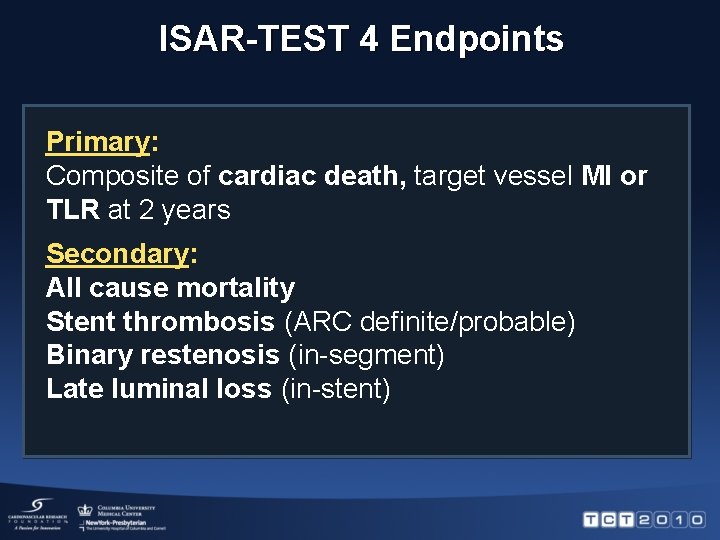
ISAR-TEST 4 Endpoints Primary: Composite of cardiac death, target vessel MI or TLR at 2 years Secondary: All cause mortality Stent thrombosis (ARC definite/probable) Binary restenosis (in-segment) Late luminal loss (in-stent)

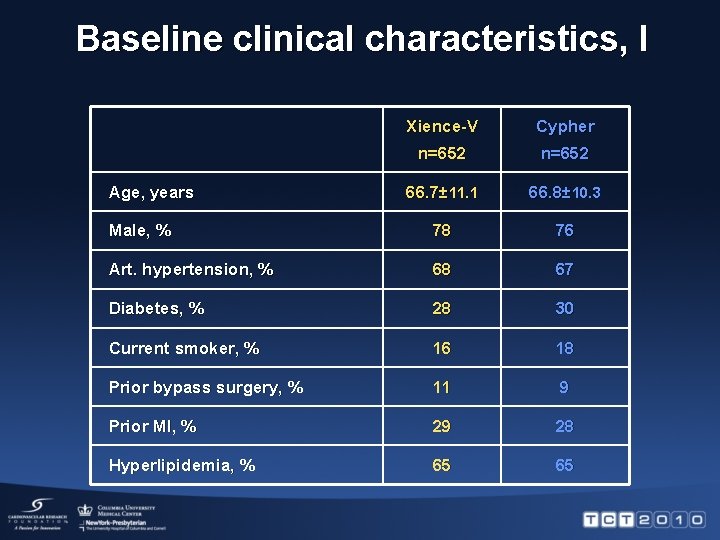
Baseline clinical characteristics, I Xience-V Cypher n=652 66. 7± 11. 1 66. 8± 10. 3 Male, % 78 76 Art. hypertension, % 68 67 Diabetes, % 28 30 Current smoker, % 16 18 Prior bypass surgery, % 11 9 Prior MI, % 29 28 Hyperlipidemia, % 65 65 Age, years

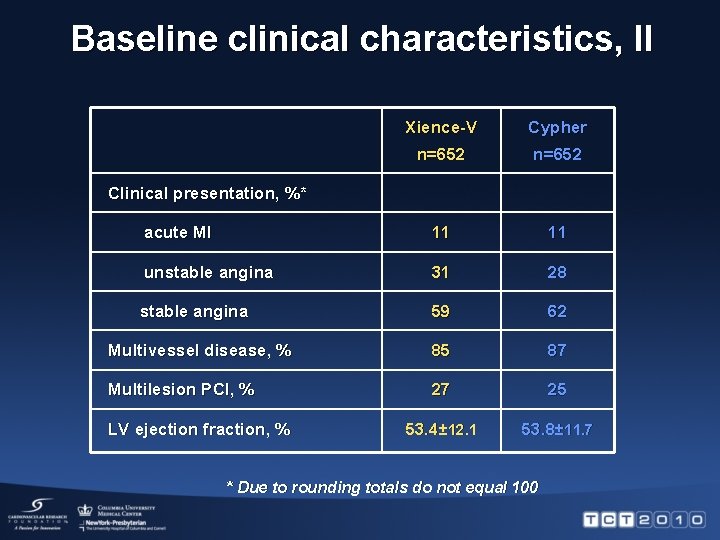
Baseline clinical characteristics, II Xience-V Cypher n=652 acute MI 11 11 unstable angina 31 28 stable angina 59 62 Multivessel disease, % 85 87 Multilesion PCI, % 27 25 53. 4± 12. 1 53. 8± 11. 7 Clinical presentation, %* LV ejection fraction, % * Due to rounding totals do not equal 100

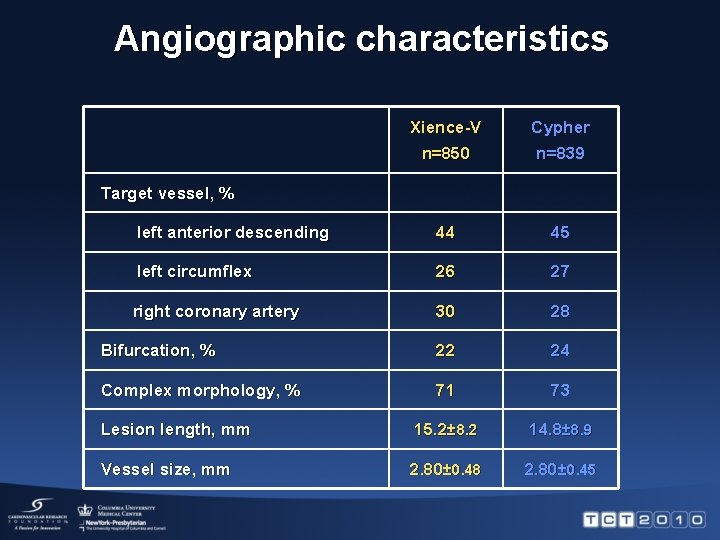
Angiographic characteristics Xience-V Cypher n=850 n=839 left anterior descending 44 45 left circumflex 26 27 right coronary artery 30 28 Bifurcation, % 22 24 Complex morphology, % 71 73 Lesion length, mm 15. 2± 8. 2 14. 8± 8. 9 Vessel size, mm 2. 80± 0. 48 2. 80± 0. 45 Target vessel, %

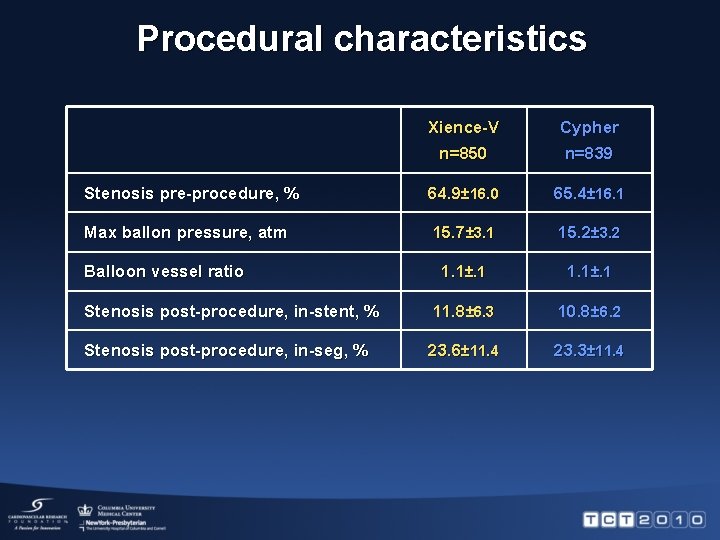
Procedural characteristics Xience-V Cypher n=850 n=839 Stenosis pre-procedure, % 64. 9± 16. 0 65. 4± 16. 1 Max ballon pressure, atm 15. 7± 3. 1 15. 2± 3. 2 1. 1±. 1 Stenosis post-procedure, in-stent, % 11. 8± 6. 3 10. 8± 6. 2 Stenosis post-procedure, in-seg, % 23. 6± 11. 4 23. 3± 11. 4 Balloon vessel ratio

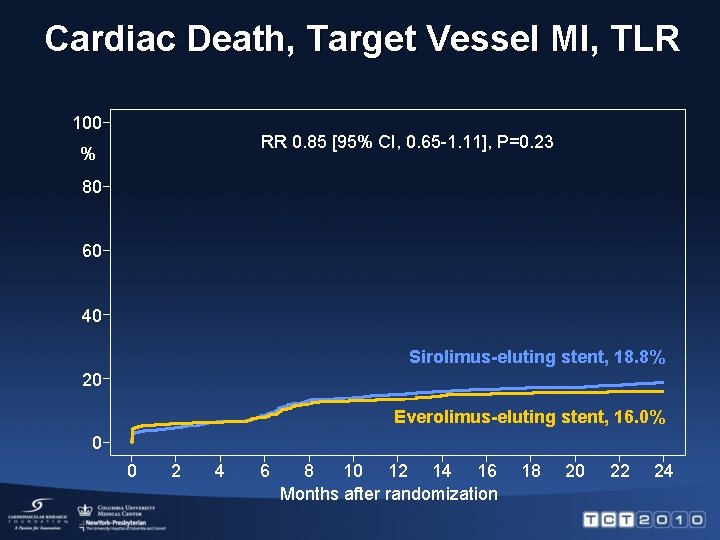
Cardiac Death, Target Vessel MI, TLR 100 RR 0. 85 [95% CI, 0. 65 -1. 11], P=0. 23 % 80 60 40 Sirolimus-eluting stent, 18. 8% 20 Everolimus-eluting stent, 16. 0% 0 0 2 4 6 8 10 12 14 16 Months after randomization 18 20 22 24
![All Cause Death 10 RR 0 93 95 CI 0 61 1 43 P0 All Cause Death 10 RR 0. 93 [95% CI, 0. 61 -1. 43]; P=0.](https://slidetodoc.com/presentation_image_h/96cc846388df962c5e9ea0a5fdbfaf91/image-14.jpg)
All Cause Death 10 RR 0. 93 [95% CI, 0. 61 -1. 43]; P=0. 75 % 8 Sirolimus-eluting stent, 6. 7% 6 Everolimus-eluting stent, 6. 4% 4 2 0 0 2 4 6 8 10 12 14 16 Months after randomization 18 20 22 24

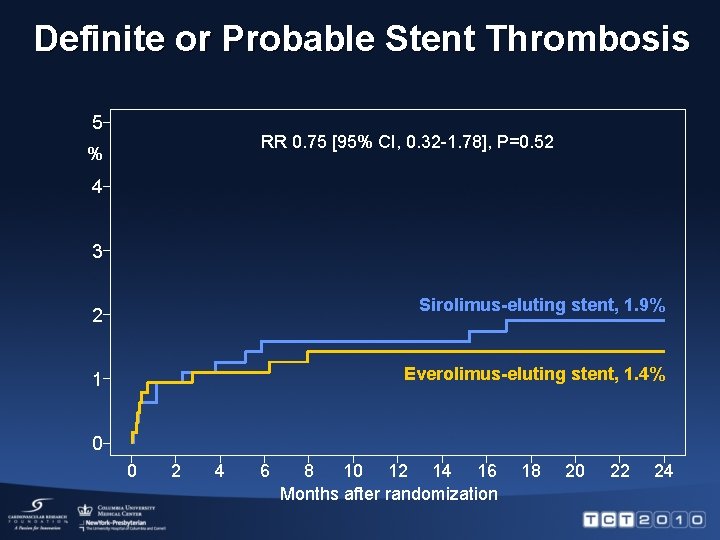
Definite or Probable Stent Thrombosis 5 RR 0. 75 [95% CI, 0. 32 -1. 78], P=0. 52 % 4 3 Sirolimus-eluting stent, 1. 9% 2 Everolimus-eluting stent, 1. 4% 1 0 0 2 4 6 8 10 12 14 16 Months after randomization 18 20 22 24

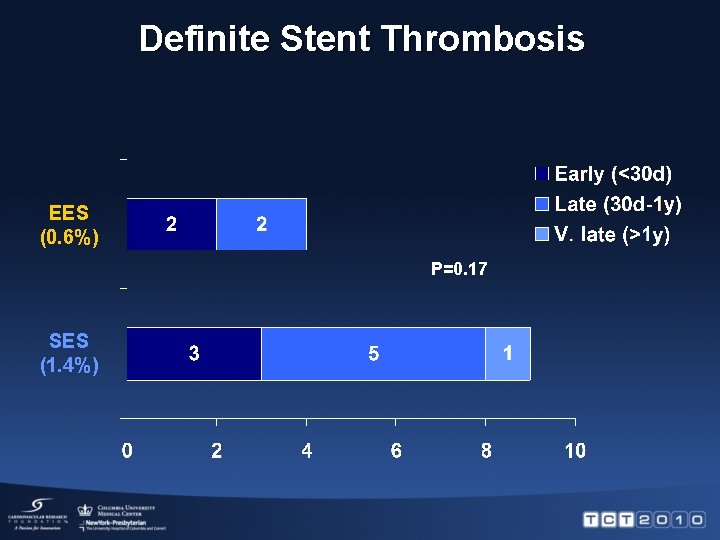
Definite Stent Thrombosis EES (0. 6%) P=0. 17 SES (1. 4%)
![Target Lesion Revascularization 100 RR 0 73 95 CI 0 52 1 01 P0 Target Lesion Revascularization 100 RR 0. 73 [95% CI, 0. 52 -1. 01], P=0.](https://slidetodoc.com/presentation_image_h/96cc846388df962c5e9ea0a5fdbfaf91/image-17.jpg)
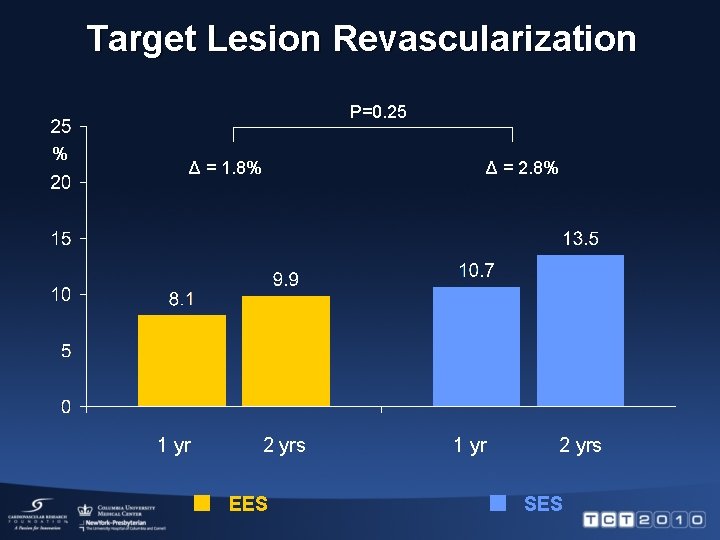
Target Lesion Revascularization 100 RR 0. 73 [95% CI, 0. 52 -1. 01], P=0. 06 % 80 60 40 Sirolimus-eluting stent, 13. 5% 20 Everolimus-eluting stent, 9. 9% 0 0 2 4 6 8 10 12 14 16 Months after randomization 18 20 22 24

Target Lesion Revascularization P=0. 25 % Δ = 1. 8% 1 yr Δ = 2. 8% 2 yrs EES 1 yr 2 yrs SES

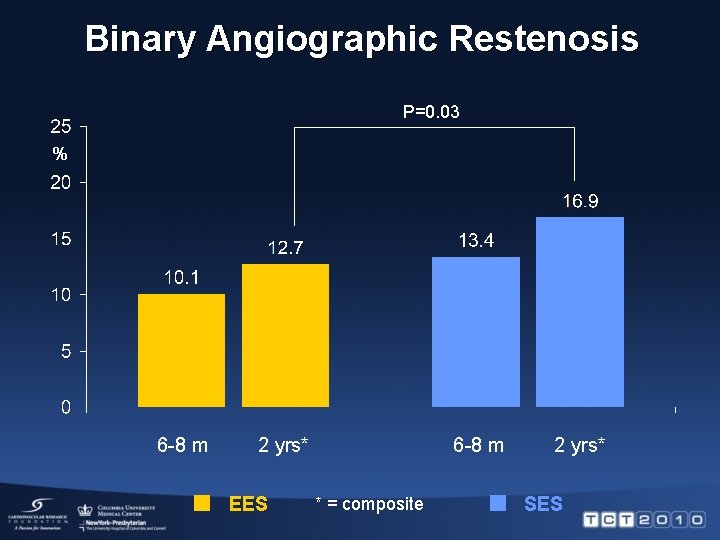
Binary Angiographic Restenosis P=0. 03 % 6 -8 m 2 yrs* EES 6 -8 m * = composite 2 yrs* SES

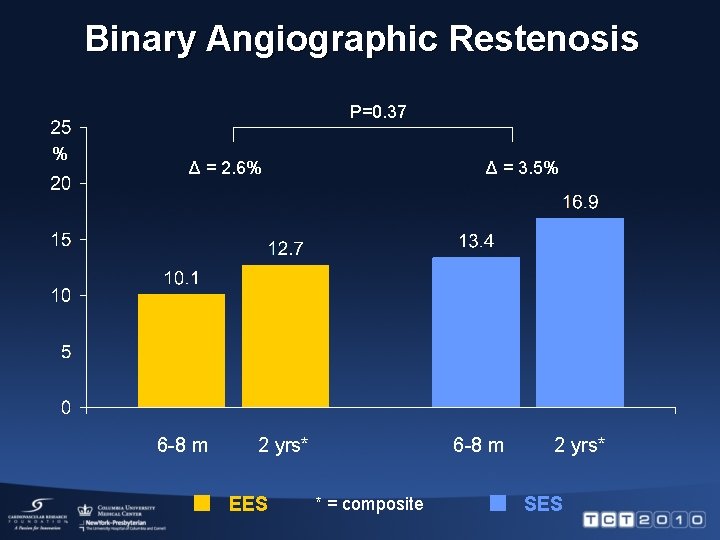
Binary Angiographic Restenosis P=0. 37 % Δ = 2. 6% 6 -8 m Δ = 3. 5% 2 yrs* EES 6 -8 m * = composite 2 yrs* SES

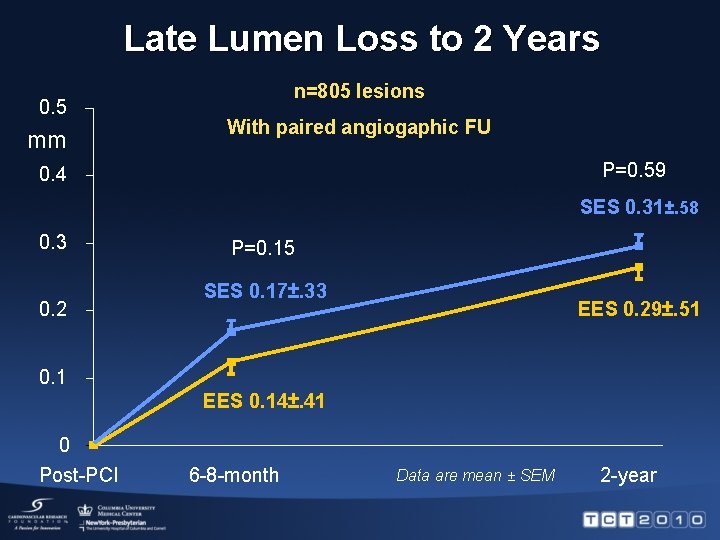
Late Lumen Loss to 2 Years 0. 5 mm n=805 lesions With paired angiogaphic FU P=0. 59 0. 4 SES 0. 31±. 58 0. 3 0. 2 P=0. 15 SES 0. 17±. 33 EES 0. 29±. 51 0. 1 EES 0. 14±. 41 0 Post-PCI 6 -8 -month Data are mean ± SEM 2 -year

Conclusions Ø In a randomized clinical trial with broad inclusion criteria, EES (Xience) and SES (Cypher) provide comparable clinical outcomes out to 2 years Ø While there was a trend towards superior antirestenotic efficacy with EES (Xience), specifically-powered studies are needed to evaluate the clinical significance of this finding

Thank You ISAR-TEST-4 Deutsches Herzzentrum, Munich. Germany
 Sirolimus rapamune
Sirolimus rapamune Sirolimus rapamune
Sirolimus rapamune Antipychotics
Antipychotics After me after me after me
After me after me after me After me after me after me
After me after me after me Sales and revenue are the same
Sales and revenue are the same Learning outcomes of ascending and descending order
Learning outcomes of ascending and descending order Objective of rhymes
Objective of rhymes Fundamental counting principle examples
Fundamental counting principle examples Learning outcome generator
Learning outcome generator Positive practice positive outcomes
Positive practice positive outcomes Examples of learning outcomes
Examples of learning outcomes Course objectives of database management systems
Course objectives of database management systems Examples of ifsp outcomes and strategies
Examples of ifsp outcomes and strategies Triangle consulting social enterprise ltd
Triangle consulting social enterprise ltd Observational medical outcomes partnership
Observational medical outcomes partnership Learning outcomes examples english
Learning outcomes examples english Outcomes focused regulation
Outcomes focused regulation Expected results research proposal example
Expected results research proposal example Workshop outcomes examples
Workshop outcomes examples Learning objectives of work and energy
Learning objectives of work and energy Relationship between aims and objectives
Relationship between aims and objectives Venn diagram of ncbts and ppst
Venn diagram of ncbts and ppst Learning outcomes of email writing
Learning outcomes of email writing













































