Thinking of Now and Tomorrow Emerging Treatment Paradigms







- Slides: 32

Thinking of Now and Tomorrow: Emerging Treatment Paradigms Pedro Cahn

Disclosures • Advisory boards: Merck, Vii. V Healthcare • Research funds: Abbvie, Merck, Richmond, Vii. V Healthcare • Speaker at educational activities: Abbvie, Gilead, Merck, Vii. V Healthcare

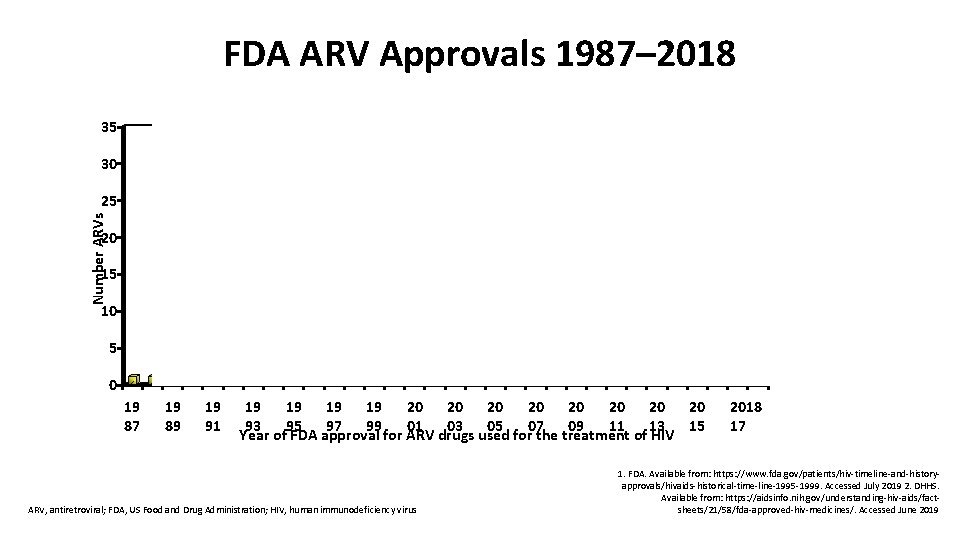
FDA ARV Approvals 1987– 2018 BIC IBA DOR 35 30 Number ARVs 25 20 15 10 5 dd. C dd. I AZT NFV DLV APV TDF RTV EFV LPV/r IDV ABC NVP 3 TC SQV d 4 T ETR RAL ENF MVC ATV DRV FTC TPV FPV RPV DTG TAF EVG 0 19 87 19 89 19 91 19 19 20 20 93 95 97 99 01 03 05 07 09 11 13 15 Year of FDA approval for ARV drugs used for the treatment of HIV ARV, antiretroviral; FDA, US Food and Drug Administration; HIV, human immunodeficiency virus 2018 20 17 1. FDA. Available from: https: //www. fda. gov/patients/hiv-timeline-and-historyapprovals/hivaids-historical-time-line-1995 -1999. Accessed July 2019 2. DHHS. Available from: https: //aidsinfo. nih. gov/understanding-hiv-aids/factsheets/21/58/fda-approved-hiv-medicines/. Accessed June 2019

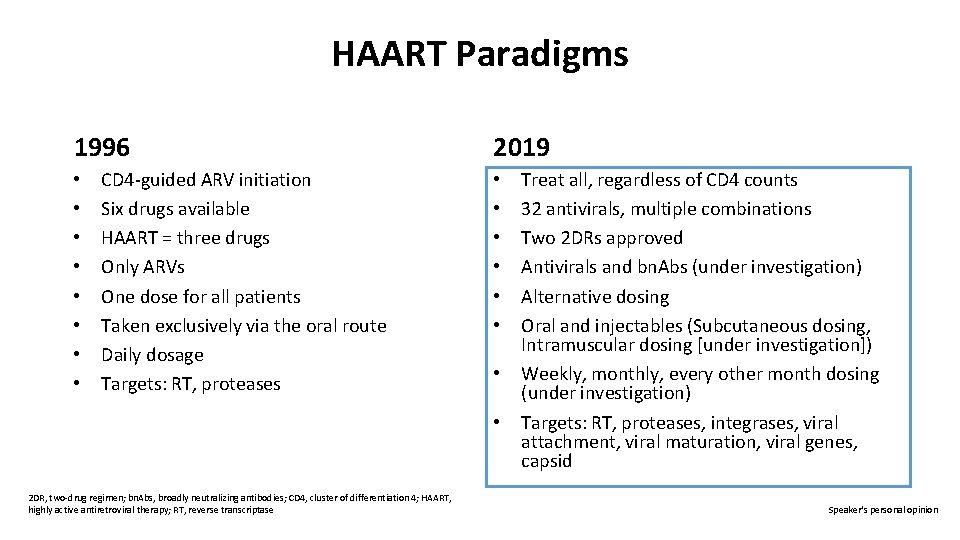
HAART Paradigms 1996 • • CD 4 -guided ARV initiation Six drugs available HAART = three drugs Only ARVs One dose for all patients Taken exclusively via the oral route Daily dosage Targets: RT, proteases 2019 • • 2 DR, two-drug regimen; bn. Abs, broadly neutralizing antibodies; CD 4, cluster of differentiation 4; HAART, highly active antiretroviral therapy; RT, reverse transcriptase Treat all, regardless of CD 4 counts 32 antivirals, multiple combinations Two 2 DRs approved Antivirals and bn. Abs (under investigation) Alternative dosing Oral and injectables (Subcutaneous dosing, Intramuscular dosing [under investigation]) Weekly, monthly, every other month dosing (under investigation) Targets: RT, proteases, integrases, viral attachment, viral maturation, viral genes, capsid Speaker's personal opinion

What is the Role of EFV in 2019? • RAL: superior at 5 years (exploratory analysis) • DTG: superior at 48 weeks (primary endpoint) • RPV: non-inferior at 48 weeks (superior in patients with BL p. VL <100, 000 c/m. L) • DOR: non-inferior at 48 weeks and better tolerated • Impact on the CNS: – – based on the ACTG meta-analysis of four studies risk of suicidality dyslipidemia increasing rates of primary resistance ACTG, AIDS Clinical Trials Group; CNS, central nervous system; DOR, doravirine; DTG, dolutegravir; EFV, efavirenz; RAL, raltegravir; RNA, ribonucleic acid; RPV, rilpivirine

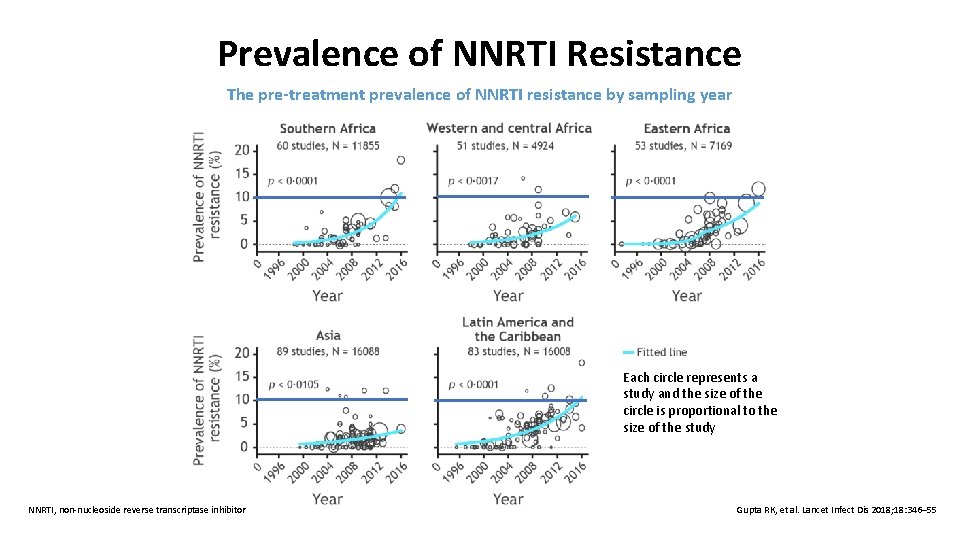
Prevalence of NNRTI Resistance The pre-treatment prevalence of NNRTI resistance by sampling year Each circle represents a study and the size of the circle is proportional to the size of the study NNRTI, non-nucleoside reverse transcriptase inhibitor Gupta RK, et al. Lancet Infect Dis 2018; 18: 346– 55

Simplification Strategies • • • Reduce number of doses a day Reduce number of pills Reduce drug dosage Reduce number of drugs Reduce number of days on ART Expand dosing interval ART, antiretroviral therapy Speaker's personal opinion

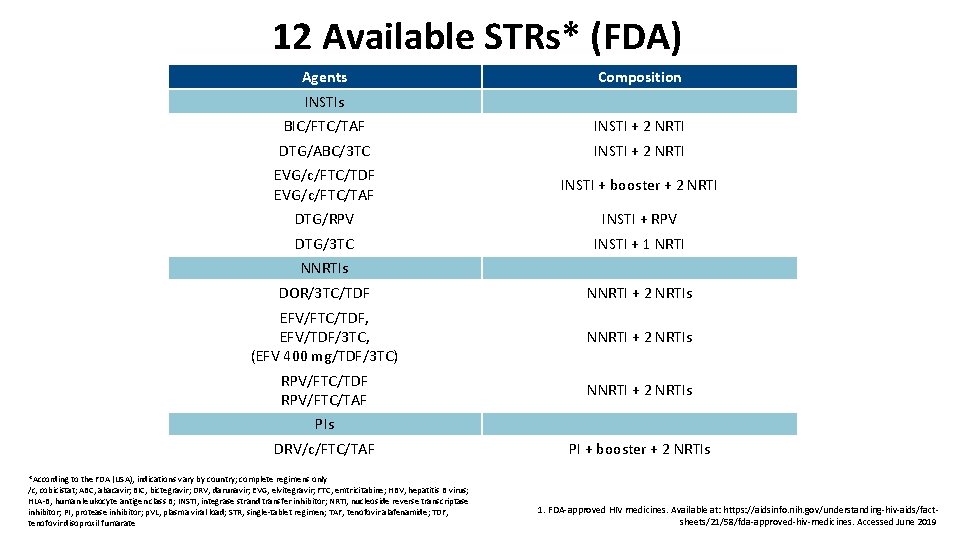
12 Available STRs* (FDA) Agents Composition INSTIs BIC/FTC/TAF INSTI + 2 NRTI DTG/ABC/3 TC INSTI + 2 NRTI EVG/c/FTC/TDF EVG/c/FTC/TAF INSTI + booster + 2 NRTI DTG/RPV INSTI + RPV DTG/3 TC INSTI + 1 NRTI NNRTIs DOR/3 TC/TDF NNRTI + 2 NRTIs EFV/FTC/TDF, EFV/TDF/3 TC, (EFV 400 mg/TDF/3 TC) NNRTI + 2 NRTIs RPV/FTC/TDF RPV/FTC/TAF NNRTI + 2 NRTIs PIs DRV/c/FTC/TAF *According to the FDA (USA), indications vary by country; complete regimens only /c, cobicistat; ABC, abacavir; BIC, bictegravir; DRV, darunavir; EVG, elvitegravir; FTC, emtricitabine; HBV, hepatitis B virus; HLA-B, human leukocyte antigen class B; INSTI, integrase strand transfer inhibitor; NRTI, nucleoside reverse transcriptase inhibitor; PI, protease inhibitor; p. VL, plasma viral load; STR, single-tablet regimen; TAF, tenofovir alafenamide; TDF, tenofovir disoproxil fumarate PI + booster + 2 NRTIs 1. FDA-approved HIV medicines. Available at: https: //aidsinfo. nih. gov/understanding-hiv-aids/factsheets/21/58/fda-approved-hiv-medicines. Accessed June 2019

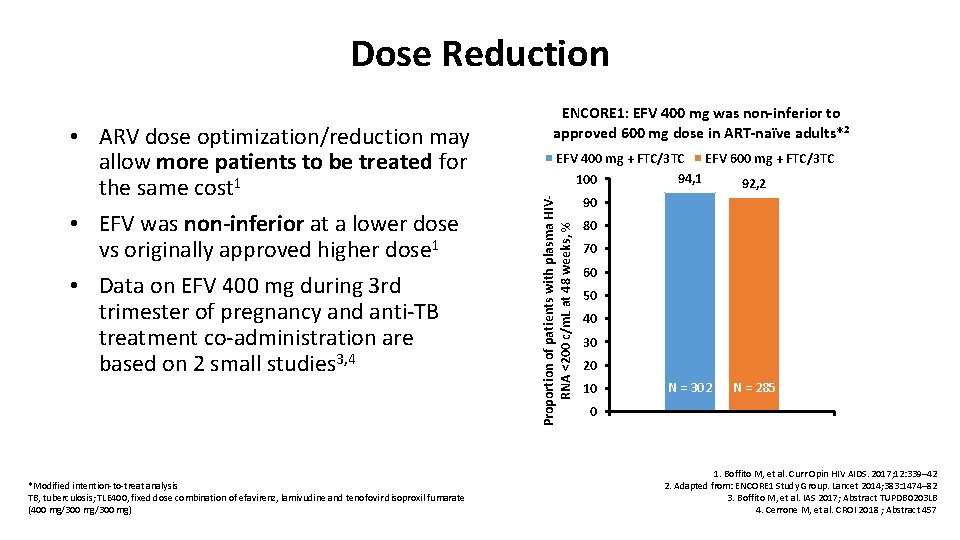
Dose Reduction *Modified intention-to-treat analysis TB, tuberculosis; TLE 400, fixed dose combination of efavirenz, lamivudine and tenofovir disoproxil fumarate (400 mg/300 mg) EFV 400 mg + FTC/3 TC EFV 600 mg + FTC/3 TC 94, 1 100 92, 2 Proportion of patients with plasma HIVRNA <200 c/m. L at 48 weeks, % • ARV dose optimization/reduction may allow more patients to be treated for the same cost 1 • EFV was non-inferior at a lower dose vs originally approved higher dose 1 • Data on EFV 400 mg during 3 rd trimester of pregnancy and anti-TB treatment co-administration are based on 2 small studies 3, 4 ENCORE 1: EFV 400 mg was non-inferior to approved 600 mg dose in ART-naïve adults*2 90 80 70 60 50 40 30 20 10 N = 302 N = 285 0 1. Boffito M, et al. Curr Opin HIV AIDS. 2017; 12: 339– 42 2. Adapted from: ENCORE 1 Study Group. Lancet 2014; 383: 1474– 82 3. Boffito M, et al. IAS 2017; Abstract TUPDB 0203 LB 4. Cerrone M, et al. CROI 2018 ; Abstract 457

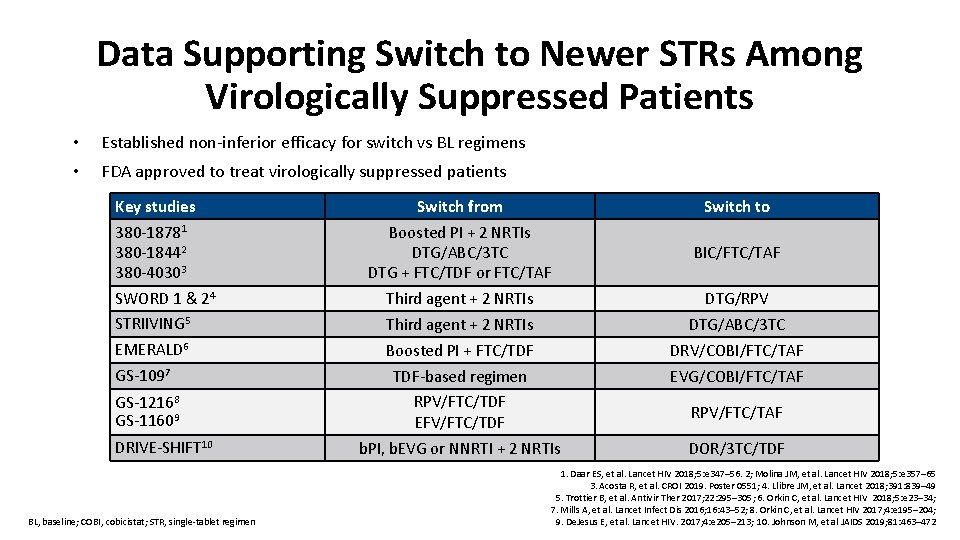
Data Supporting Switch to Newer STRs Among Virologically Suppressed Patients • Established non-inferior efficacy for switch vs BL regimens • FDA approved to treat virologically suppressed patients Key studies Switch from Switch to Boosted PI + 2 NRTIs DTG/ABC/3 TC DTG + FTC/TDF or FTC/TAF BIC/FTC/TAF Third agent + 2 NRTIs DTG/RPV STRIIVING 5 Third agent + 2 NRTIs DTG/ABC/3 TC EMERALD 6 Boosted PI + FTC/TDF DRV/COBI/FTC/TAF GS-1097 TDF-based regimen EVG/COBI/FTC/TAF GS-12168 GS-11609 RPV/FTC/TDF EFV/FTC/TDF RPV/FTC/TAF b. PI, b. EVG or NNRTI + 2 NRTIs DOR/3 TC/TDF 380 -18781 380 -18442 380 -40303 SWORD 1 & 24 DRIVE-SHIFT 10 BL, baseline; COBI, cobicistat; STR, single-tablet regimen 1. Daar ES, et al. Lancet HIV 2018; 5: e 347– 56. 2; Molina JM, et al. Lancet HIV 2018; 5: e 357– 65 3. Acosta R, et al. CROI 2019. Poster 0551; 4. Llibre JM, et al. Lancet 2018; 391: 839– 49 5. Trottier B, et al. Antivir Ther 2017; 22: 295– 305; 6. Orkin C, et al. Lancet HIV 2018; 5: e 23– 34; 7. Mills A, et al. Lancet Infect Dis 2016; 16: 43– 52; 8. Orkin C, et al. Lancet HIV 2017; 4: e 195– 204; 9. De. Jesus E, et al. Lancet HIV. 2017; 4: e 205– 213; 10. Johnson M, et al JAIDS 2019; 81: 463– 472

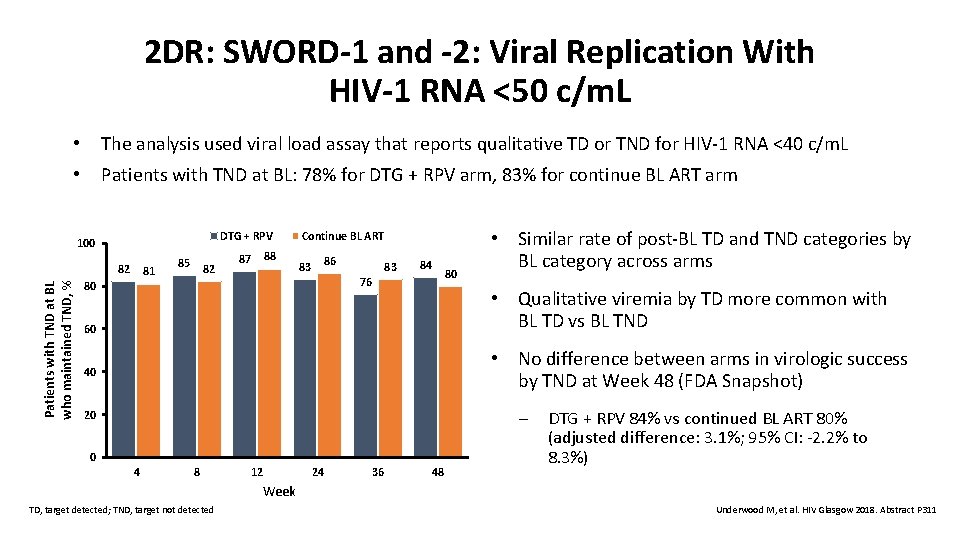
2 DR: SWORD-1 and -2: Viral Replication With HIV-1 RNA <50 c/m. L • The analysis used viral load assay that reports qualitative TD or TND for HIV-1 RNA <40 c/m. L • Patients with TND at BL: 78% for DTG + RPV arm, 83% for continue BL ART arm DTG + RPV 100 Patients with TND at BL who maintained TND, % 82 81 85 82 88 87 Continue BL ART 86 83 83 84 80 76 80 • Similar rate of post-BL TD and TND categories by BL category across arms • Qualitative viremia by TD more common with BL TD vs BL TND 60 • No difference between arms in virologic success by TND at Week 48 (FDA Snapshot) 40 – 20 0 4 8 12 24 36 48 DTG + RPV 84% vs continued BL ART 80% (adjusted difference: 3. 1%; 95% CI: -2. 2% to 8. 3%) Week TD, target detected; TND, target not detected Underwood M, et al. HIV Glasgow 2018. Abstract P 311

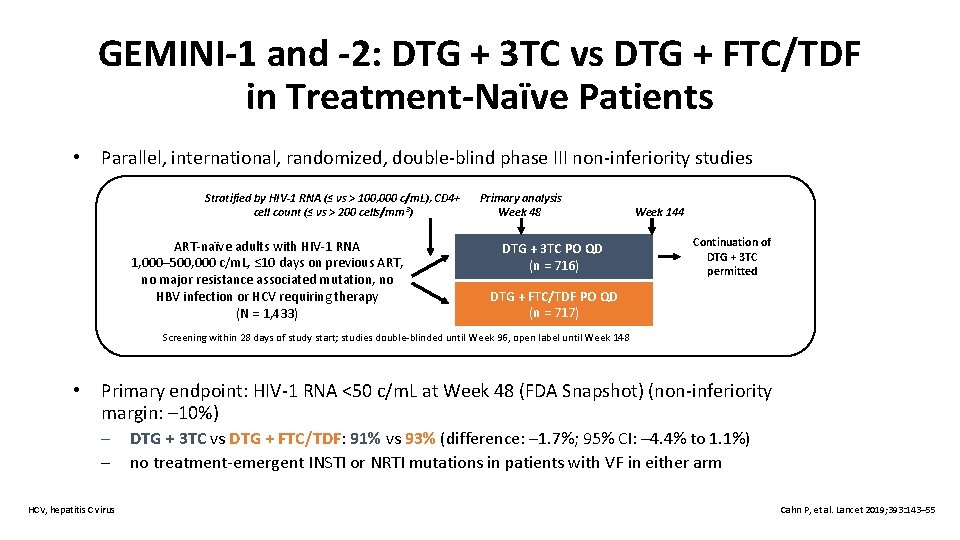
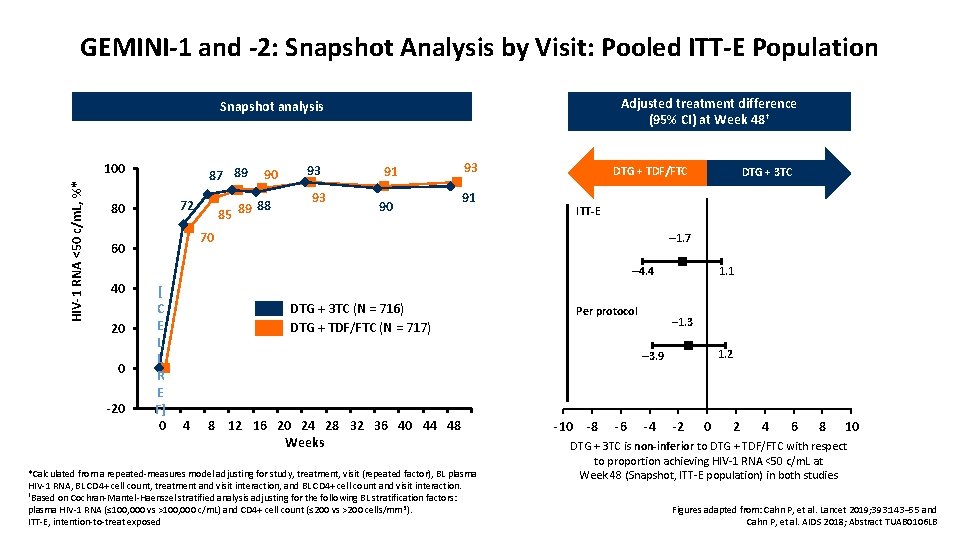
GEMINI-1 and -2: DTG + 3 TC vs DTG + FTC/TDF in Treatment-Naïve Patients • Parallel, international, randomized, double-blind phase III non-inferiority studies Stratified by HIV-1 RNA (≤ vs > 100, 000 c/m. L), CD 4+ cell count (≤ vs > 200 cells/mm³) ART-naïve adults with HIV-1 RNA 1, 000– 500, 000 c/m. L, ≤ 10 days on previous ART, no major resistance associated mutation, no HBV infection or HCV requiring therapy (N = 1, 433) Primary analysis Week 48 DTG + 3 TC PO QD (n = 716) Week 144 Continuation of DTG + 3 TC permitted DTG + FTC/TDF PO QD (n = 717) Screening within 28 days of study start; studies double-blinded until Week 96, open label until Week 148 • Primary endpoint: HIV-1 RNA <50 c/m. L at Week 48 (FDA Snapshot) (non-inferiority margin: – 10%) – – HCV, hepatitis C virus DTG + 3 TC vs DTG + FTC/TDF: 91% vs 93% (difference: – 1. 7%; 95% CI: – 4. 4% to 1. 1%) no treatment-emergent INSTI or NRTI mutations in patients with VF in either arm Cahn P, et al. Lancet 2019; 393: 143– 55

GEMINI-1 and -2: Snapshot Analysis by Visit: Pooled ITT-E Population Adjusted treatment difference (95% CI) at Week 48† Snapshot analysis HIV-1 RNA <50 c/m. L, %* 100 80 87 89 72 90 88 85 89 93 93 91 90 93 91 DTG + TDF/FTC ITT-E 70 60 DTG + 3 TC – 1. 7 1. 1 – 4. 4 40 [ C E 20 L L 0 R E -20 F] -4 0 DTG + 3 TC (N = 716) DTG + TDF/FTC (N = 717) Per protocol – 1. 3 1. 2 – 3. 9 4 8 12 16 20 24 28 32 36 40 44 48 Weeks *Calculated from a repeated-measures model adjusting for study, treatment, visit (repeated factor), BL plasma HIV-1 RNA, BL CD 4+ cell count, treatment and visit interaction, and BL CD 4+ cell count and visit interaction. †Based on Cochran-Mantel-Haenszel stratified analysis adjusting for the following BL stratification factors: plasma HIV-1 RNA (≤ 100, 000 vs >100, 000 c/m. L) and CD 4+ cell count (≤ 200 vs >200 cells/mm 3). ITT-E, intention-to-treat exposed -10 -8 -6 -4 -2 0 2 4 6 8 10 DTG + 3 TC is non-inferior to DTG + TDF/FTC with respect to proportion achieving HIV-1 RNA <50 c/m. L at Week 48 (Snapshot, ITT-E population) in both studies Figures adapted from: Cahn P, et al. Lancet 2019; 393: 143– 55 and Cahn P, et al. AIDS 2018; Abstract TUAB 0106 LB

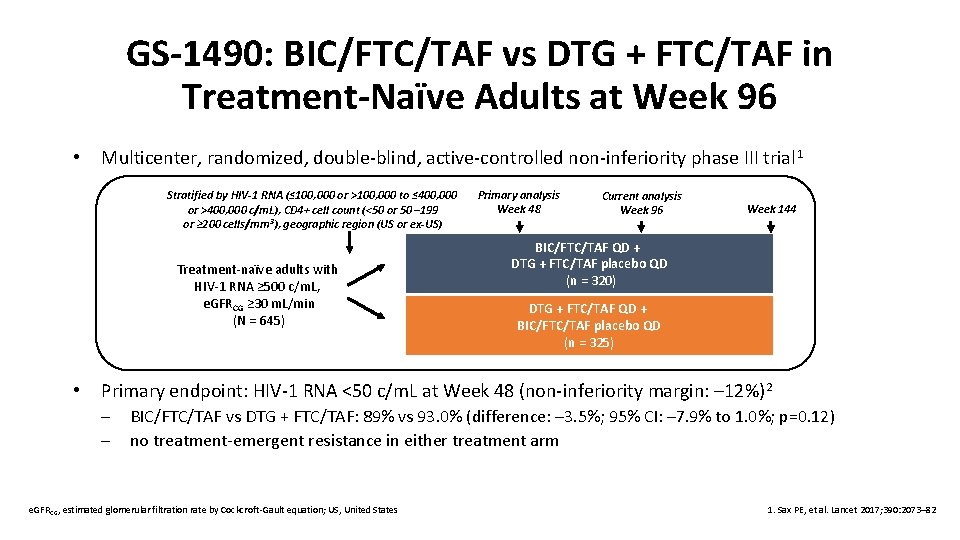
GS-1490: BIC/FTC/TAF vs DTG + FTC/TAF in Treatment-Naïve Adults at Week 96 • Multicenter, randomized, double-blind, active-controlled non-inferiority phase III trial 1 Stratified by HIV-1 RNA (≤ 100, 000 or >100, 000 to ≤ 400, 000 or >400, 000 c/m. L), CD 4+ cell count (<50 or 50 – 199 or ≥ 200 cells/mm 3), geographic region (US or ex-US) Treatment-naïve adults with HIV-1 RNA ≥ 500 c/m. L, e. GFRCG ≥ 30 m. L/min (N = 645) • Primary analysis Week 48 Current analysis Week 96 Week 144 BIC/FTC/TAF QD + DTG + FTC/TAF placebo QD (n = 320) DTG + FTC/TAF QD + BIC/FTC/TAF placebo QD (n = 325) Primary endpoint: HIV-1 RNA <50 c/m. L at Week 48 (non-inferiority margin: – 12%) 2 – – BIC/FTC/TAF vs DTG + FTC/TAF: 89% vs 93. 0% (difference: – 3. 5%; 95% CI: – 7. 9% to 1. 0%; p=0. 12) no treatment-emergent resistance in either treatment arm e. GFRCG, estimated glomerular filtration rate by Cockcroft-Gault equation; US, United States 1. Sax PE, et al. Lancet 2017; 390: 2073– 82

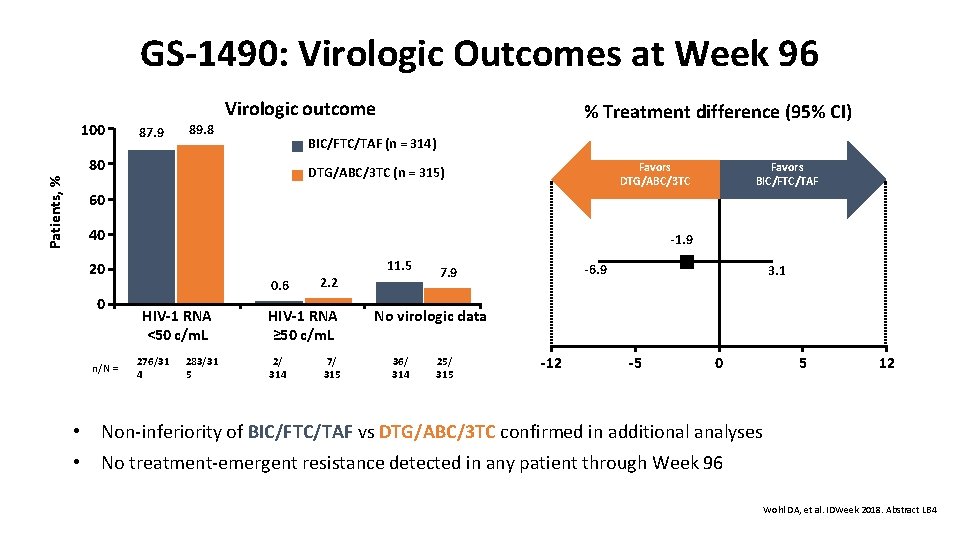
GS-1490: Virologic Outcomes at Week 96 100 Virologic outcome 87. 9 89. 8 BIC/FTC/TAF (n = 314) 80 Patients, % % Treatment difference (95% CI) Favors DTG/ABC/3 TC (n = 315) Favors BIC/FTC/TAF 60 40 -1. 9 20 0 n/N = 0. 6 HIV-1 RNA <50 c/m. L 276/31 4 283/31 5 2. 2 HIV-1 RNA ≥ 50 c/m. L 2/ 314 7/ 315 11. 5 -6. 9 7. 9 3. 1 No virologic data 36/ 314 25/ 315 -12 -5 0 • Non-inferiority of BIC/FTC/TAF vs DTG/ABC/3 TC confirmed in additional analyses • No treatment-emergent resistance detected in any patient through Week 96 5 12 Wohl DA, et al. IDWeek 2018. Abstract LB 4

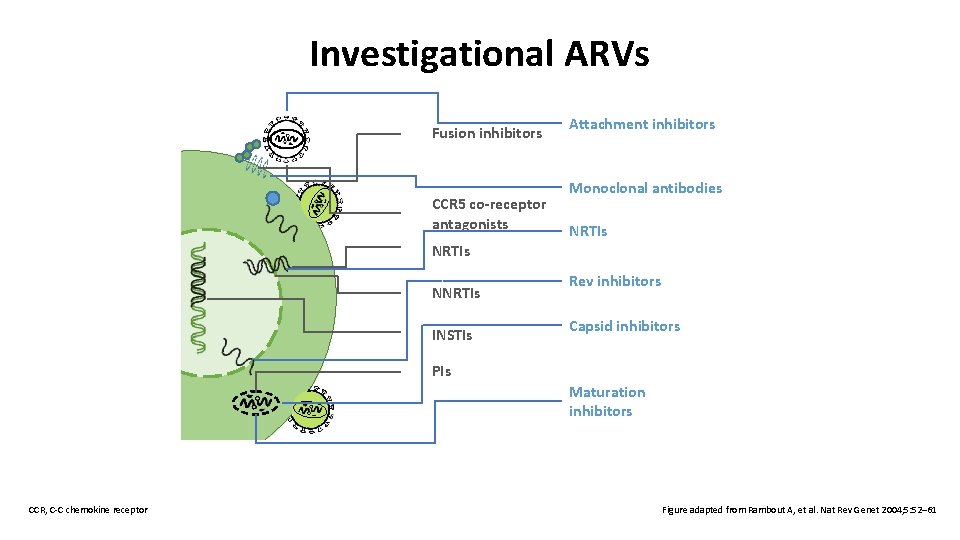
Investigational ARVs Fusion inhibitors CCR 5 co-receptor antagonists Attachment inhibitors Monoclonal antibodies NRTIs NNRTIs INSTIs Rev inhibitors Capsid inhibitors PIs Maturation inhibitors CCR, C-C chemokine receptor Figure adapted from Rambout A, et al. Nat Rev Genet 2004; 5: 52– 61

Entry Inhibitors gp 120 binding Coreceptor binding CD 4 binding CCR 5 antagonist Maraviroc Ibalizumab Fostemsavir gp 41 Virus-cell fusion Enfuvirtide gp 120 V 3 loop CD 4 Cell membrane * = FDA approved CCR 5/CXCR 4 (R 5/X 4) Figure adapted from Moore JP & Doms RW. Proc Natl Acad Sci U S A 2003; 100: 10598– 602

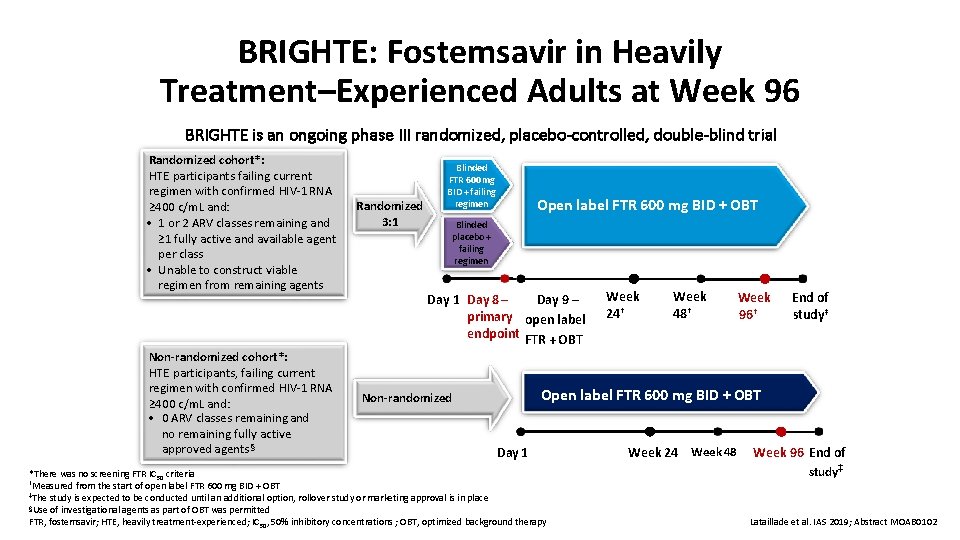
BRIGHTE: Fostemsavir in Heavily Treatment–Experienced Adults at Week 96 BRIGHTE is an ongoing phase III randomized, placebo-controlled, double-blind trial Randomized cohort*: HTE participants failing current regimen with confirmed HIV-1 RNA ≥ 400 c/m. L and: • 1 or 2 ARV classes remaining and ≥ 1 fully active and available agent per class • Unable to construct viable regimen from remaining agents Non-randomized cohort*: HTE participants, failing current regimen with confirmed HIV-1 RNA ≥ 400 c/m. L and: • 0 ARV classes remaining and no remaining fully active approved agents§ Randomized 3: 1 Blinded FTR 600 mg BID + failing regimen Open label FTR 600 mg BID + OBT Blinded placebo + failing regimen Day 9 – Day 1 Day 8 – primary open label endpoint FTR + OBT Week 24† Week 48† Week 96† End of study‡ Open label FTR 600 mg BID + OBT Non-randomized Day 1 *There was no screening FTR IC 50 criteria †Measured from the start of open label FTR 600 mg BID + OBT ‡The study is expected to be conducted until an additional option, rollover study or marketing approval is in place §Use of investigational agents as part of OBT was permitted FTR, fostemsavir; HTE, heavily treatment-experienced; IC 50, 50% inhibitory concentrations ; OBT, optimized background therapy Week 24 Week 48 Week 96 End of study ‡ Lataillade et al. IAS 2019; Abstract MOAB 0102

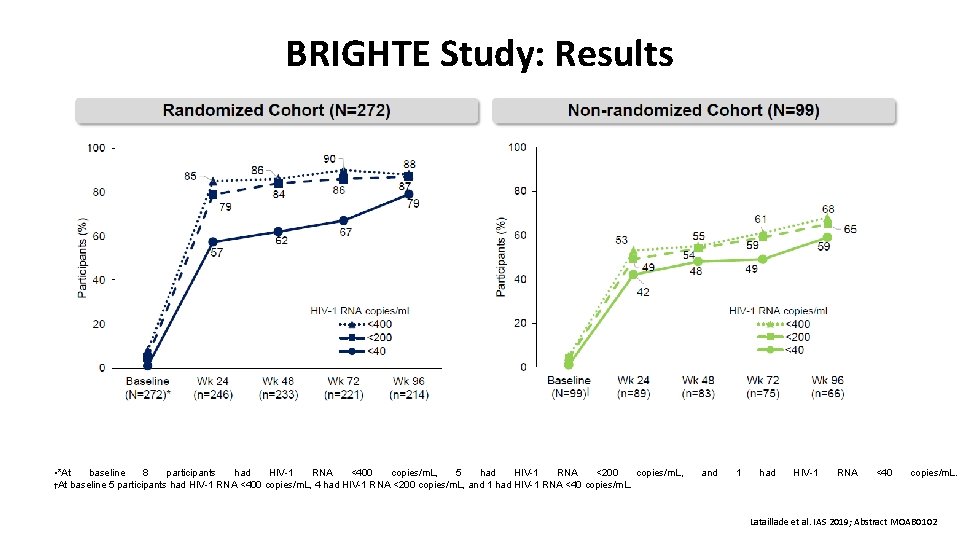
BRIGHTE Study: Results • *At baseline 8 participants had HIV-1 RNA <400 copies/m. L, 5 had HIV-1 RNA <200 copies/m. L, †At baseline 5 participants had HIV-1 RNA <400 copies/m. L, 4 had HIV-1 RNA <200 copies/m. L, and 1 had HIV-1 RNA <40 copies/m. L. Lataillade et al. IAS 2019; Abstract MOAB 0102

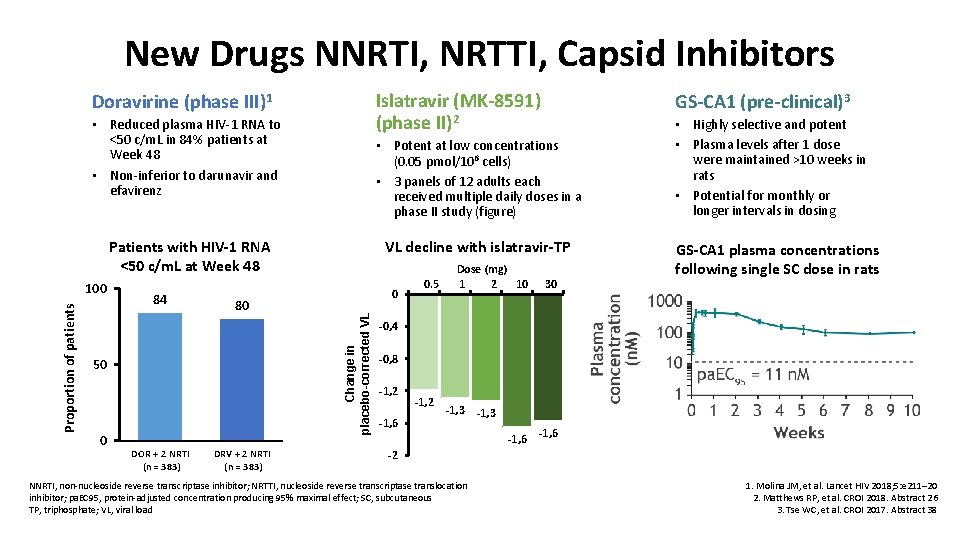
New Drugs NNRTI, NRTTI, Capsid Inhibitors Islatravir (MK-8591) (phase II)2 Doravirine (phase III)1 • Reduced plasma HIV-1 RNA to <50 c/m. L in 84% patients at Week 48 • Non-inferior to darunavir and efavirenz • Potent at low concentrations (0. 05 pmol/106 cells) • 3 panels of 12 adults each received multiple daily doses in a phase II study (figure) Patients with HIV-1 RNA <50 c/m. L at Week 48 84 80 50 0 DOR + 2 NRTI (n = 383) DRV + 2 NRTI (n = 383) VL decline with islatravir-TP 0 Change in placebo-corrected VL Proportion of patients 100 GS-CA 1 (pre-clinical)3 0. 5 Dose (mg) 1 2 10 30 • Highly selective and potent • Plasma levels after 1 dose were maintained >10 weeks in rats • Potential for monthly or longer intervals in dosing GS-CA 1 plasma concentrations following single SC dose in rats -0, 4 -0, 8 -1, 2 -1, 6 -1, 2 -1, 3 -1, 6 -2 NNRTI, non-nucleoside reverse transcriptase inhibitor; NRTTI, nucleoside reverse transcriptase translocation inhibitor; pa. EC 95, protein-adjusted concentration producing 95% maximal effect; SC, subcutaneous TP, triphosphate; VL, viral load 1. Molina JM, et al. Lancet HIV 2018; 5: e 211– 20 2. Matthews RP, et al. CROI 2018. Abstract 26 3. Tse WC, et al. CROI 2017. Abstract 38

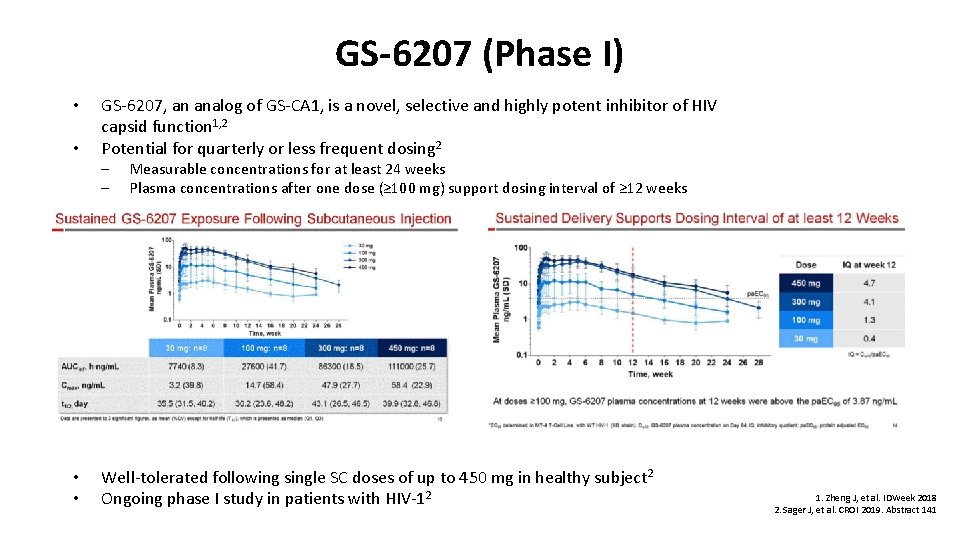
GS-6207 (Phase I) • • GS-6207, an analog of GS-CA 1, is a novel, selective and highly potent inhibitor of HIV capsid function 1, 2 Potential for quarterly or less frequent dosing 2 – – • • Measurable concentrations for at least 24 weeks Plasma concentrations after one dose (≥ 100 mg) support dosing interval of ≥ 12 weeks Well-tolerated following single SC doses of up to 450 mg in healthy subject 2 Ongoing phase I study in patients with HIV-12 1. Zheng J, et al. IDWeek 2018 2. Sager J, et al. CROI 2019. Abstract 141

Why Investigate LA/ER? Advantages • Infrequent dosing – A long apparent T½ • Lower drug dose needed (nanoformulation) • Prevents poor adherence • Possibility of directly observed therapy • Tissue targeting (LN/macrophage uptake) • Use in patients with pill fatigue • Better protection of health privacy • Avoids treatment-related HIV stigma Disadvantages • Injection site reactions • More frequent visits to the clinic • Increased HCP workload • Long tail, in case of unexpected toxicities • No chelation available, in case of urgent need Personal communication with Dr Charles Flexner ER, extended release; HCP, healthcare provider; LA, long-acting; LN, lymph node

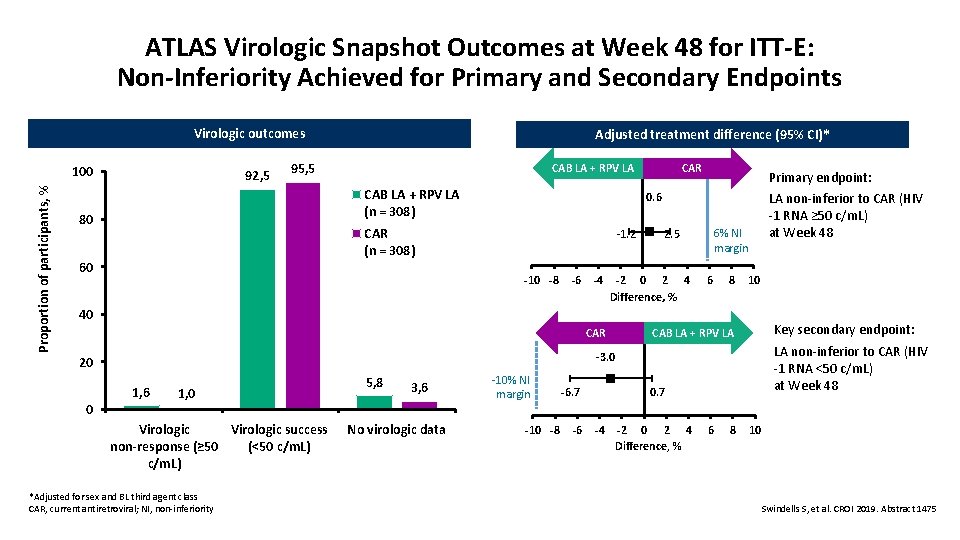
ATLAS Virologic Snapshot Outcomes at Week 48 for ITT-E: Non-Inferiority Achieved for Primary and Secondary Endpoints Virologic outcomes Proportion of participants, % 100 92, 5 Adjusted treatment difference (95% CI)* 95, 5 CAB LA + RPV LA (n = 308) 80 Primary endpoint: LA non-inferior to CAR (HIV -1 RNA ≥ 50 c/m. L) at Week 48 0. 6 CAR (n = 308) 60 CAR CAB LA + RPV LA -1. 2 -10 -8 -6 -4 6% NI margin 2. 5 -2 0 2 4 Difference, % 6 8 10 40 CAR Key secondary endpoint: 0. 7 LA non-inferior to CAR (HIV -1 RNA <50 c/m. L) at Week 48 -3. 0 20 1, 6 0 CAB LA + RPV LA 1, 0 Virologic success Virologic (<50 c/m. L) non-response (≥ 50 c/m. L) *Adjusted for sex and BL third agent class CAR, current antiretroviral; NI, non-inferiority 5, 8 3, 6 No virologic data -10% NI margin -10 -8 -6. 7 -6 -4 -2 0 2 4 Difference, % 6 8 10 Swindells S, et al. CROI 2019. Abstract 1475

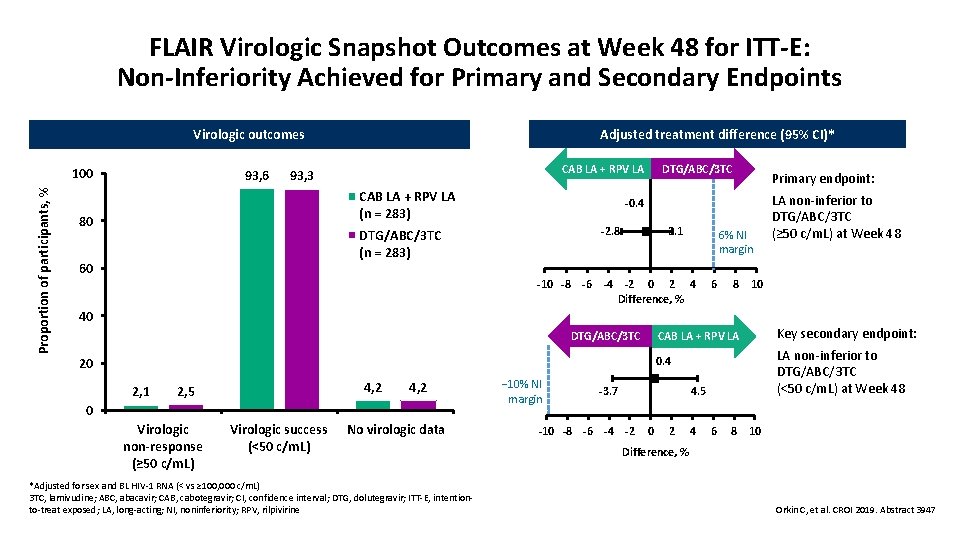
FLAIR Virologic Snapshot Outcomes at Week 48 for ITT-E: Non-Inferiority Achieved for Primary and Secondary Endpoints Adjusted treatment difference (95% CI)* Virologic outcomes Proportion of participants, % 100 93, 6 CAB LA + RPV LA (n = 283) 80 Primary endpoint: -0. 4 -2. 8 DTG/ABC/3 TC (n = 283) 60 DTG/ABC/3 TC CAB LA + RPV LA 93, 3 2. 1 6% NI margin -10 -8 -6 -4 -2 0 2 4 Difference, % 6 LA non-inferior to DTG/ABC/3 TC (≥ 50 c/m. L) at Week 48 8 10 40 DTG/ABC/3 TC CAB LA + RPV LA LA non-inferior to DTG/ABC/3 TC (<50 c/m. L) at Week 48 0. 4 20 2, 1 4, 2 2, 5 4, 2 0 Virologic non-response (≥ 50 c/m. L) Virologic success (<50 c/m. L) No virologic data *Adjusted for sex and BL HIV-1 RNA (< vs ≥ 100, 000 c/m. L) 3 TC, lamivudine; ABC, abacavir; CAB, cabotegravir; CI, confidence interval; DTG, dolutegravir; ITT-E, intentionto-treat exposed; LA, long-acting; NI, noninferiority; RPV, rilpivirine − 10% NI margin -3. 7 4. 5 -10 -8 -6 -4 -2 0 2 4 Key secondary endpoint: 6 8 10 Difference, % Orkin C, et al. CROI 2019. Abstract 3947

Long-Acting ARV Implants • Potential advantages over injectable therapy – – Removable More consistent and predictable drug release PK not dependent on injection site May remain in place for years (inert, non-degradable SC versions) • Potential disadvantages over injectables – – PK, pharmacokinetic Specialized device required for insertion Minor surgical procedure to remove Regulated as both a drug and a device Potential difficulties moving to a generic marketplace Personal communication with Dr Charles Flexner

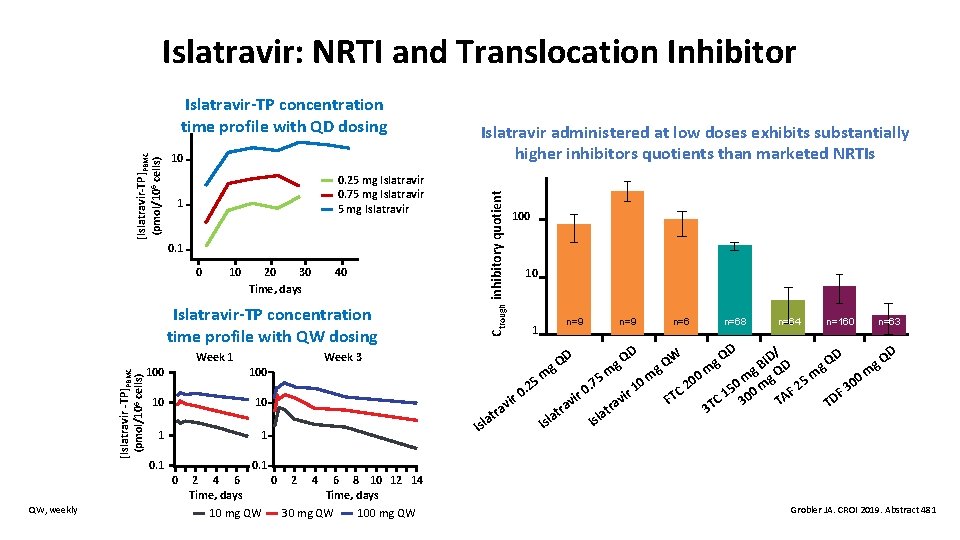
Islatravir: NRTI and Translocation Inhibitor 10 0. 25 mg Islatravir 0. 75 mg Islatravir 1 0 10 20 30 Time, days 40 Islatravir-TP concentration time profile with QW dosing [Islatravir -TP]PBMC (pmol/106 cells) Week 1 100 10 10 1 1 0. 1 100 10 1 n=9 n=68 D n=64 n=160 n=63 D D/ QD Q g D g g m Q m m 0 m m m 5 0 m g 0 5 5 7 0 0 0. 2 2 m 2 1 0 F 0. 15 00 F 3 ir TC A ir r D v C i F v 3 T T v 3 T tra ra a t a l l a Is Is Isl D g. Q QW g. Q I g. B 0. 1 0 QW, weekly Week 3 Islatravir administered at low doses exhibits substantially higher inhibitors quotients than marketed NRTIs Ctrough inhibitory quotient [Islatravir-TP]PBMC (pmol/106 cells) Islatravir-TP concentration time profile with QD dosing 2 4 6 Time, days 10 mg QW 0 2 4 6 8 10 12 14 Time, days 30 mg QW 100 mg QW Grobler JA. CROI 2019. Abstract 481

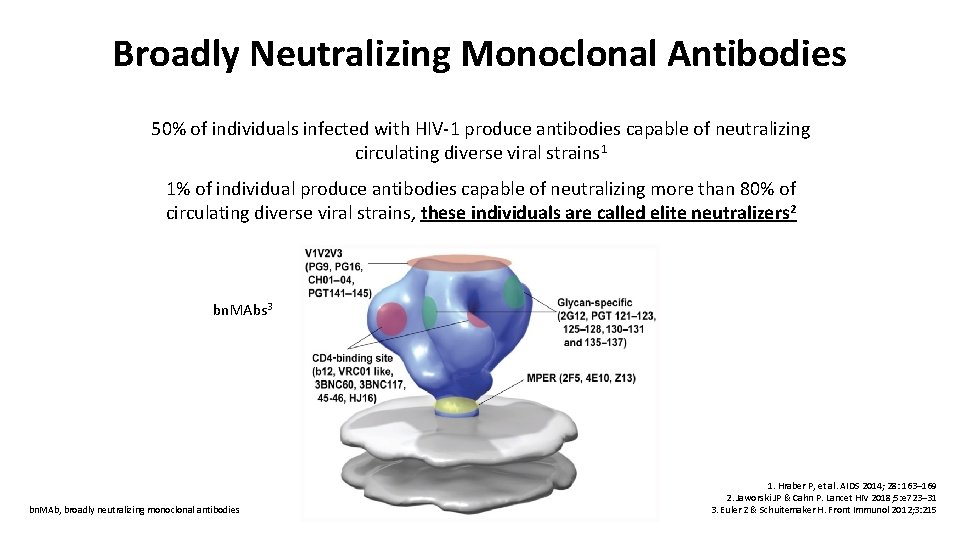
Broadly Neutralizing Monoclonal Antibodies 50% of individuals infected with HIV-1 produce antibodies capable of neutralizing circulating diverse viral strains 1 1% of individual produce antibodies capable of neutralizing more than 80% of circulating diverse viral strains, these individuals are called elite neutralizers 2 bn. MAbs 3 bn. MAb, broadly neutralizing monoclonal antibodies 1. Hraber P, et al. AIDS 2014; 28: 163– 169 2. Jaworski JP & Cahn P. Lancet HIV 2018; 5: e 723– 31 3. Euler Z & Schuitemaker H. Front Immunol 2012; 3: 215

Early Treatment With bn. MAbs Interferes With Viral Seeding Hyperacute infection (0– 14 days) DNA, deoxyribonucleic acid; Nm. Ab, neutralizing monoclonal antibody; PBMC, peripheral blood mononuclear cell; SIV, simian immunodeficiency virus Hessell AJ, et al. Nat Med 2016 22: 362– 8

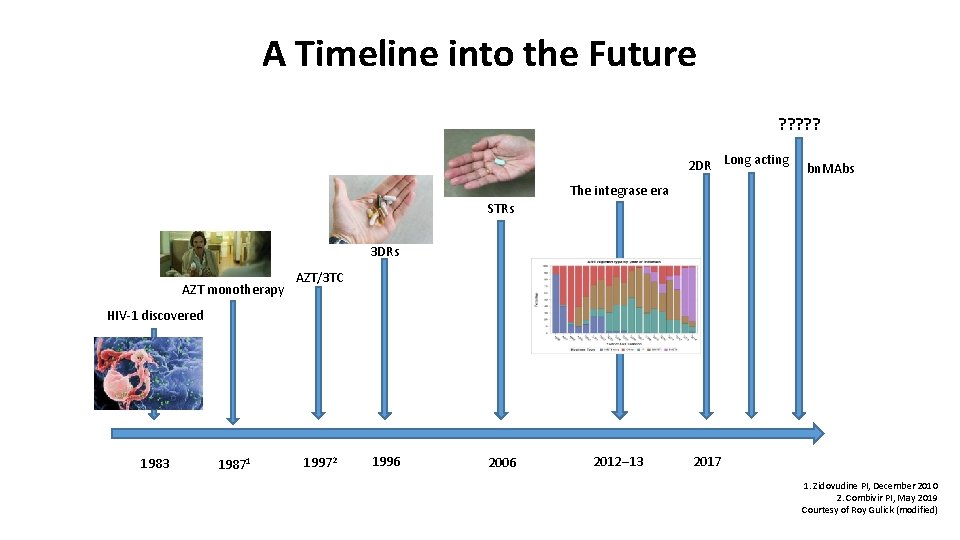
A Timeline into the Future ? ? ? 2 DR Long acting bn. MAbs The integrase era STRs 3 DRs AZT monotherapy AZT/3 TC HIV-1 discovered 1983 19871 19972 1996 2006 2012– 13 2017 1. Zidovudine PI, December 2010 2. Combivir PI, May 2019 Courtesy of Roy Gulick (modified)

Conclusions • • The effectiveness of ART is already very high The guidelines lean towards INSTIs Other classes have new combinations (TAF/FTC/DRV/c and TDF/3 TC/DOR) Patients request simpler treatments There are different strategies in development 2 DRs (already approved by FDA and EMA) Long-acting drugs Experimental: – – weekly oral doses monthly injectables implants b-n. Mabs The future looks really exciting!

Thank you for your attention! pedro. cahn@huesped. org. ar

Thank you.
 Tomorrow and tomorrow shakespeare
Tomorrow and tomorrow shakespeare Tomorrow and tomorrow and tomorrow kurt vonnegut analysis
Tomorrow and tomorrow and tomorrow kurt vonnegut analysis Due tomorrow do tomorrow
Due tomorrow do tomorrow Due tomorrow do tomorrow
Due tomorrow do tomorrow Nursing now today's issues tomorrow's trends
Nursing now today's issues tomorrow's trends Nursing now today's issues tomorrow's trends
Nursing now today's issues tomorrow's trends Now i see it now you don't
Now i see it now you don't Boyfriend/girlfriend centered paradigm
Boyfriend/girlfriend centered paradigm Paradigmatic analysis
Paradigmatic analysis Distributed systems principles and paradigms
Distributed systems principles and paradigms Distributed systems principles and paradigms
Distributed systems principles and paradigms Php features and paradigms
Php features and paradigms Paradigms and principles
Paradigms and principles Distributed systems principles and paradigms
Distributed systems principles and paradigms Distributed systems principles and paradigms
Distributed systems principles and paradigms Positive thinking vs negative thinking examples
Positive thinking vs negative thinking examples Thinking about your own thinking
Thinking about your own thinking Analytic vs holistic thinking example
Analytic vs holistic thinking example Perbedaan critical thinking dan creative thinking
Perbedaan critical thinking dan creative thinking Thinking about you thinking about me
Thinking about you thinking about me Hall policy paradigms
Hall policy paradigms 4 paradigms of cognitive psychology
4 paradigms of cognitive psychology Paradigm of interaction in hci
Paradigm of interaction in hci Message ordering paradigms
Message ordering paradigms Distributed computing paradigm
Distributed computing paradigm 3 paradigms
3 paradigms Interpretivism in consumer behaviour
Interpretivism in consumer behaviour Paradigms of others examples
Paradigms of others examples Binding in programming paradigms
Binding in programming paradigms Ktu programming paradigms notes
Ktu programming paradigms notes Objectives of communication ppt
Objectives of communication ppt Multimedia application design
Multimedia application design Evaluation paradigms
Evaluation paradigms