Emerging Treatment Paradigms in NSCLC Edward S Kim


























- Slides: 66

Emerging Treatment Paradigms in NSCLC Edward S. Kim, MD MD Anderson Cancer Center

Tobacco Use in the USA 1900 -1999 Per capita cigarette consumption Male lung cancer death rate Female lung cancer death rate *Age-adjusted to 2000 US standard population. Source: Death rates: US Mortality Public Use Tapes, 1960 -1999, US Mortality Volumes, 1930 -1959, National Center for Health Statistics, Centers for Disease Control and Prevention, 2001. Cigarette consumption: Us Department of Agriculture, 1900 -1999.

Cancer Deaths in the US 80 60 †Uterine 1990 1980 Year 1970 1990 1980 1970 0 1960 0 1950 20 1940 20 1950 40 1940 40 Pancreas Liver Prostate Stomach Lung & bronchus Colon & rectum 1930 60 Rate per 100, 000 Uterus† Breast Pancreas Ovary Stomach Lung & bronchus Colon & rectum 1930 Rate per 100, 000 80 Male 1960 Female cancer death rates are for uterine cervic and uterine corpus combined. Source: US Mortality Public Use Data Tapes 1960 -1996. US Mortality Volumes 1930 -1959, National Center for Health Statistics, Centers for Disease Control and Prevention, 1999.

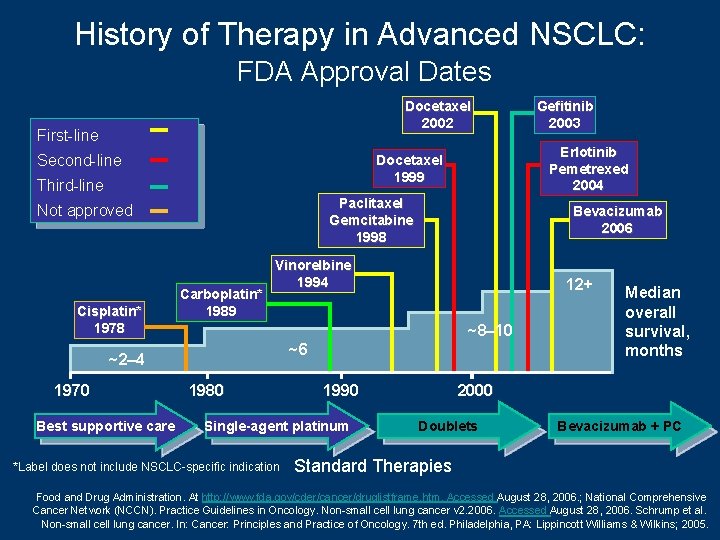
History of Therapy in Advanced NSCLC: FDA Approval Dates Docetaxel 2002 First-line Second-line Paclitaxel Gemcitabine 1998 Not approved Cisplatin* 1978 Carboplatin* 1989 Best supportive care Bevacizumab 2006 Vinorelbine 1994 12+ ~8– 10 ~6 ~2– 4 1970 Erlotinib Pemetrexed 2004 Docetaxel 1999 Third-line 1980 1990 Single-agent platinum *Label does not include NSCLC-specific indication Gefitinib 2003 Median overall survival, months 2000 Doublets Bevacizumab + PC Standard Therapies Food and Drug Administration. At http: //www. fda. gov/cder/cancer/druglistframe. htm. Accessed August 28, 2006. ; National Comprehensive Cancer Network (NCCN). Practice Guidelines in Oncology. Non-small cell lung cancer v 2. 2006. Accessed August 28, 2006. Schrump et al. Non-small cell lung cancer. In: Cancer: Principles and Practice of Oncology. 7 th ed. Philadelphia, PA: Lippincott Williams & Wilkins; 2005.

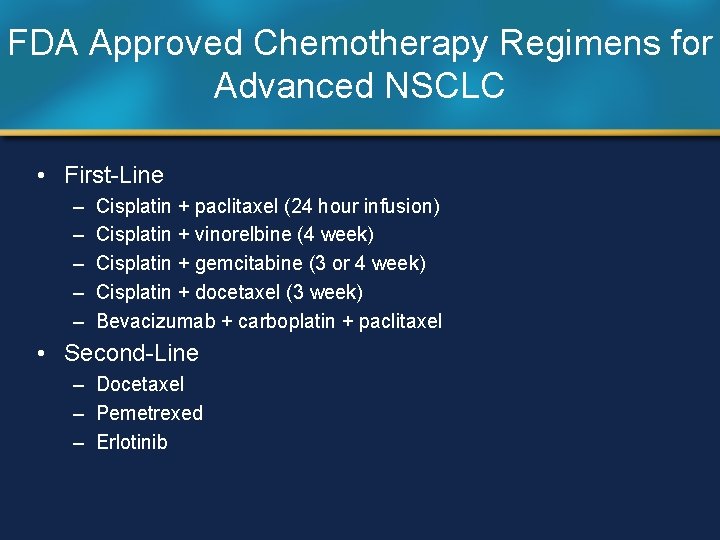
FDA Approved Chemotherapy Regimens for Advanced NSCLC • First-Line – – – Cisplatin + paclitaxel (24 hour infusion) Cisplatin + vinorelbine (4 week) Cisplatin + gemcitabine (3 or 4 week) Cisplatin + docetaxel (3 week) Bevacizumab + carboplatin + paclitaxel • Second-Line – Docetaxel – Pemetrexed – Erlotinib

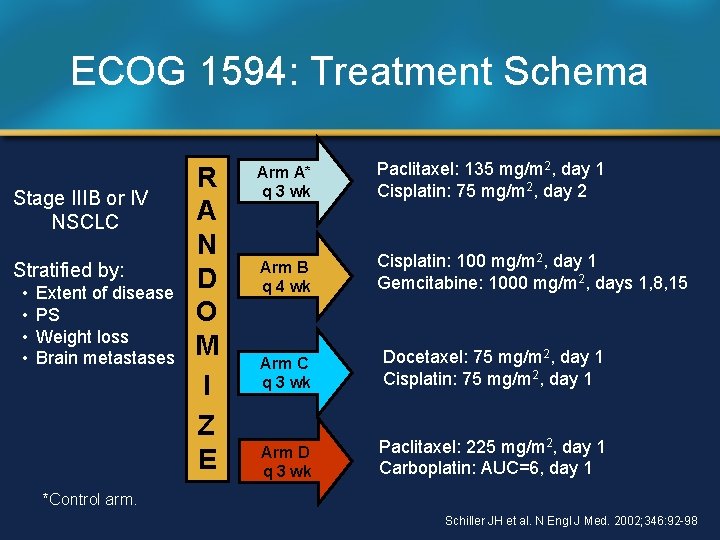
ECOG 1594: Treatment Schema Stage IIIB or IV NSCLC Stratified by: • • Extent of disease PS Weight loss Brain metastases R A N D O M I Z E Arm A* q 3 wk Paclitaxel: 135 mg/m 2, day 1 Cisplatin: 75 mg/m 2, day 2 Arm B q 4 wk Cisplatin: 100 mg/m 2, day 1 Gemcitabine: 1000 mg/m 2, days 1, 8, 15 Arm C q 3 wk Docetaxel: 75 mg/m 2, day 1 Cisplatin: 75 mg/m 2, day 1 Arm D q 3 wk Paclitaxel: 225 mg/m 2, day 1 Carboplatin: AUC=6, day 1 *Control arm. Schiller JH et al. N Engl J Med. 2002; 346: 92 -98

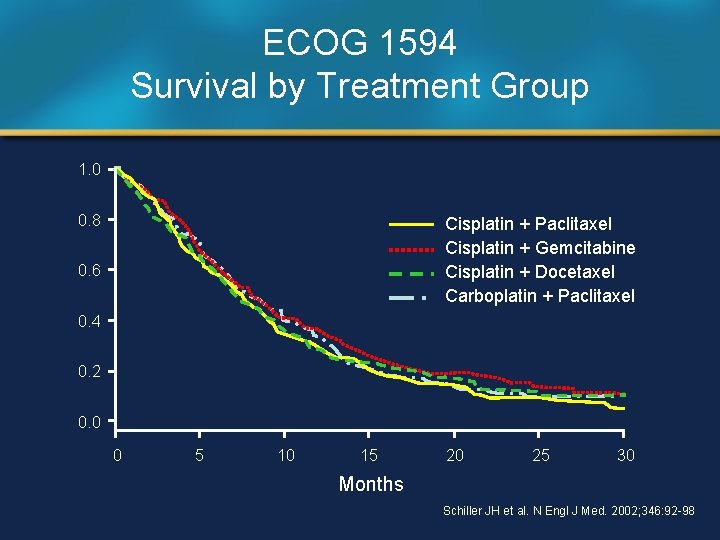
ECOG 1594 Survival by Treatment Group 1. 0 0. 8 Cisplatin + Paclitaxel Cisplatin + Gemcitabine Cisplatin + Docetaxel Carboplatin + Paclitaxel 0. 6 0. 4 0. 2 0. 0 0 5 10 15 20 25 30 Months Schiller JH et al. N Engl J Med. 2002; 346: 92 -98

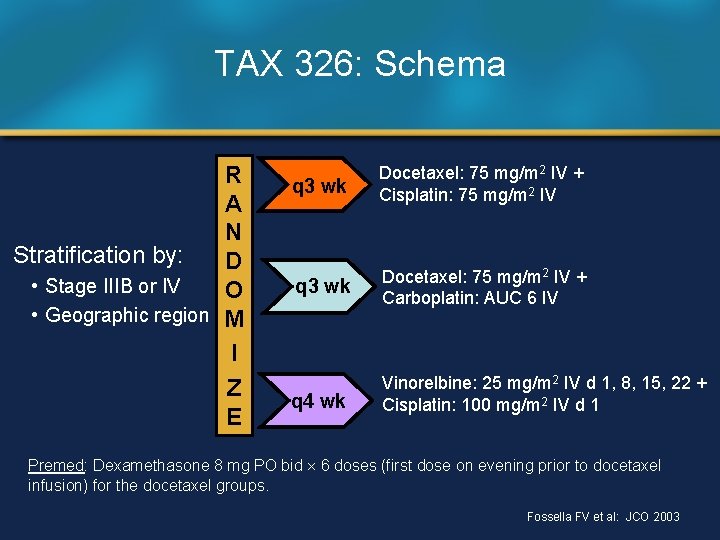
TAX 326: Schema R A N Stratification by: D • Stage IIIB or IV O • Geographic region M I Z E q 3 wk Docetaxel: 75 mg/m 2 IV + Cisplatin: 75 mg/m 2 IV q 3 wk Docetaxel: 75 mg/m 2 IV + Carboplatin: AUC 6 IV q 4 wk Vinorelbine: 25 mg/m 2 IV d 1, 8, 15, 22 + Cisplatin: 100 mg/m 2 IV d 1 Premed: Dexamethasone 8 mg PO bid 6 doses (first dose on evening prior to docetaxel infusion) for the docetaxel groups. Fossella FV et al: JCO 2003

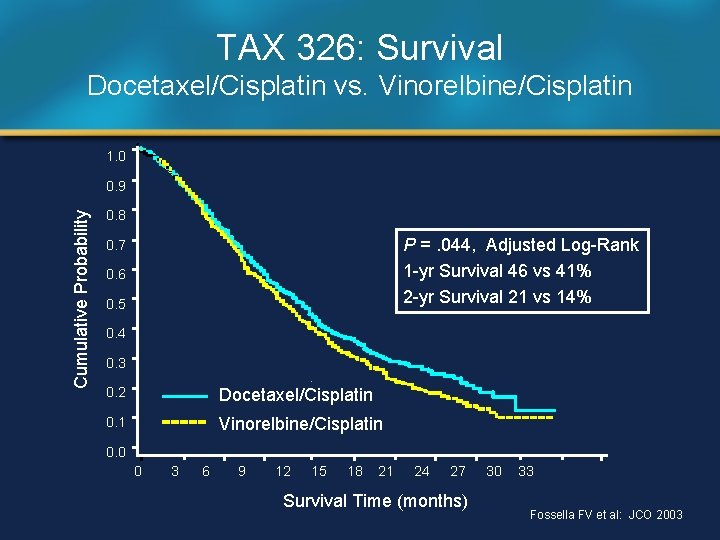
TAX 326: Survival Docetaxel/Cisplatin vs. Vinorelbine/Cisplatin 1. 0 Cumulative Probability 0. 9 0. 8 P =. 044, Adjusted Log-Rank 1 -yr Survival 46 vs 41% 2 -yr Survival 21 vs 14% 0. 7 0. 6 0. 5 0. 4 0. 3 0. 2 Docetaxel/Cisplatin 0. 1 Vinorelbine/Cisplatin 0. 0 0 3 6 9 12 15 18 21 24 27 Survival Time (months) 30 33 Fossella FV et al: JCO 2003

The Bottom Line: Metastatic NSCLC Study N ORR(%) MST (mos) SWOG 9509 Carbo-Pac Cis-Vino EGOG 1594 Cis-Pac Cis-Gem Cis-Doce Carbo-Pac 208 202 25 28 8. 0 292 288 293 290 21. 3 21 17. 3 15. 3 8. 1 7. 4 8. 3 Italian Study Cis-Gem Carbo-Pac Cis-Vino 205 201 30 32 30 9. 8 9. 9 9. 5 EORTC 08975 Cis-Pac Cis-Gem Gem-Pac 159 160 161 31 36 27 8. 1 8. 8 6. 9 TAX 326 Doce-Cis Doce-Carbo Cis-Vino 408 406 404 NA NA NA 10. 9 9. 1 10. 0

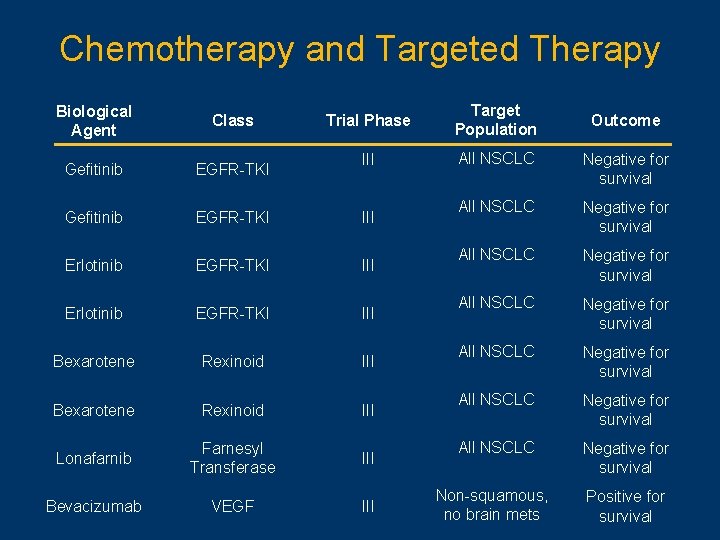
Chemotherapy and Targeted Therapy Trial Phase Target Population III All NSCLC Negative for survival All NSCLC Negative for survival Non-squamous, no brain mets Positive for survival Biological Agent Class Gefitinib EGFR-TKI III Erlotinib EGFR-TKI III Bexarotene Rexinoid III Lonafarnib Farnesyl Transferase III Bevacizumab VEGF III Outcome

Case 1: NSCLC • HPI: 76 year old female with choking episode. Heimlich maneuver x 3 successful. Hospital w/u revealed 2 cm RLL nodule. • PMH: Asthma, HTN, DM Type II, R RCCA – 1997 • Meds: HCTZ, Inderal, Nabumetone • Allergies: None • SH: Widow, cigarettes: 20 pack-years • ETOH: 3 beers/day • FH: Negative • ROS: Rib pain, unsteady gait, no weight loss • PE: No supraclavicular nodes, C/V: RRR, Resp: clear to A&P, Abd: no masses Ext: no C/C/E Neuro: no focal deficits

Case 1: NSCLC • What is the desired work-up for the nodule? 1. 2. 3. 4. Biopsy, CT chest, abdomen, bone scan, MRI brain Referral to medical oncology

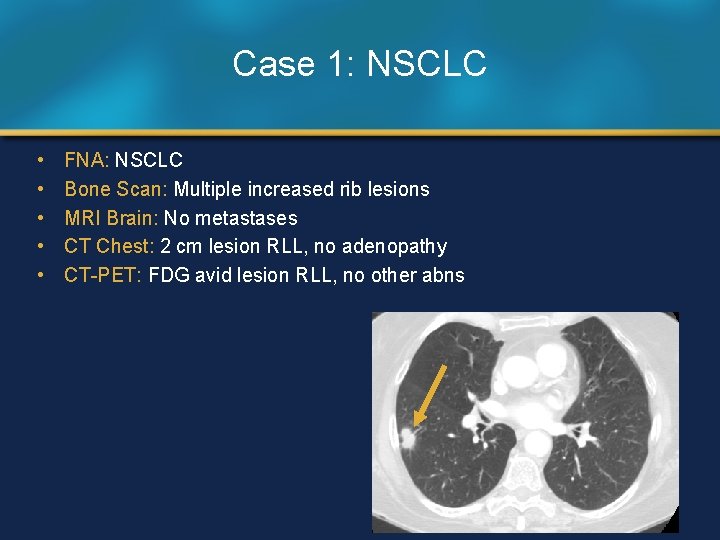
Case 1: NSCLC • • • FNA: NSCLC Bone Scan: Multiple increased rib lesions MRI Brain: No metastases CT Chest: 2 cm lesion RLL, no adenopathy CT-PET: FDG avid lesion RLL, no other abns

Case 1: NSCLC • What is the optimal treatment for this patient at this time? 1. 2. 3. 4. Lobectomy and nodal sampling Lobectomy and nodal dissection Surgery followed by radiation

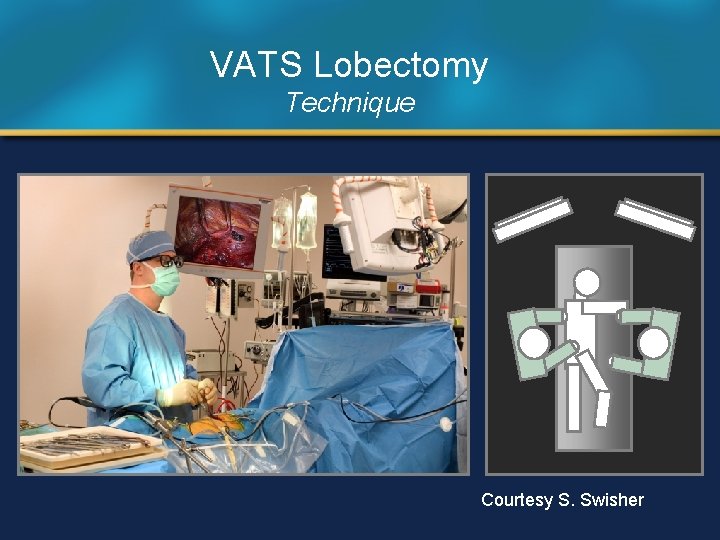
VATS Lobectomy Technique Courtesy S. Swisher

Case 1: NSCLC Pathology: T 2 N 0 M 0

Case 1: NSCLC • What is the appropriate next therapy for this patient? 1. 2. 3. 4. Adjuvant chemotherapy followed by radiation Observation Radiation alone

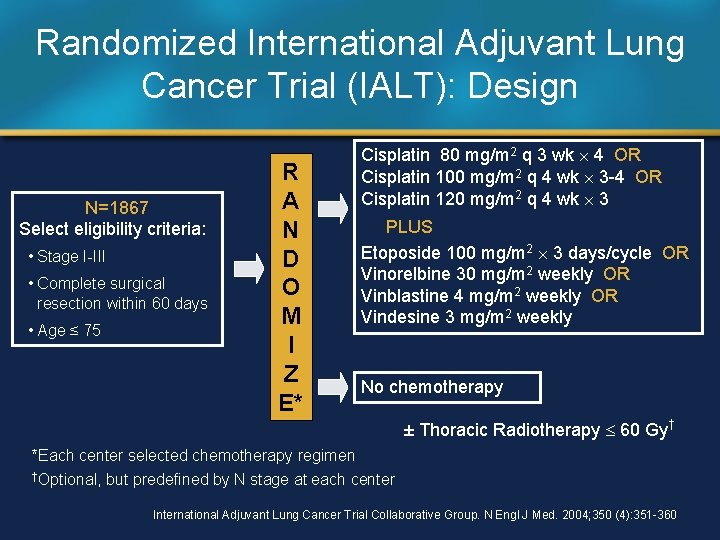
Randomized International Adjuvant Lung Cancer Trial (IALT): Design N=1867 Select eligibility criteria: • Stage I-III • Complete surgical resection within 60 days • Age ≤ 75 R A N D O M I Z E* Cisplatin 80 mg/m 2 q 3 wk 4 OR Cisplatin 100 mg/m 2 q 4 wk 3 -4 OR Cisplatin 120 mg/m 2 q 4 wk 3 PLUS Etoposide 100 mg/m 2 3 days/cycle OR Vinorelbine 30 mg/m 2 weekly OR Vinblastine 4 mg/m 2 weekly OR Vindesine 3 mg/m 2 weekly No chemotherapy ± Thoracic Radiotherapy 60 Gy† *Each center selected chemotherapy regimen †Optional, but predefined by N stage at each center International Adjuvant Lung Cancer Trial Collaborative Group. N Engl J Med. 2004; 350 (4): 351 -360

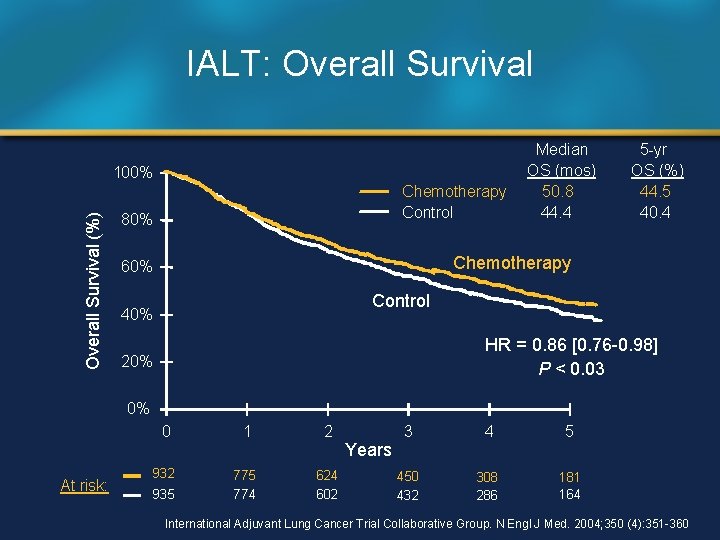
IALT: Overall Survival (%) 100% Chemotherapy Control 80% Median OS (mos) 50. 8 44. 4 5 -yr OS (%) 44. 5 40. 4 Chemotherapy 60% Control 40% HR = 0. 86 [0. 76 -0. 98] P < 0. 03 20% 0% At risk: 0 1 2 932 775 774 624 602 935 Years 3 4 5 450 432 308 286 181 164 International Adjuvant Lung Cancer Trial Collaborative Group. N Engl J Med. 2004; 350 (4): 351 -360

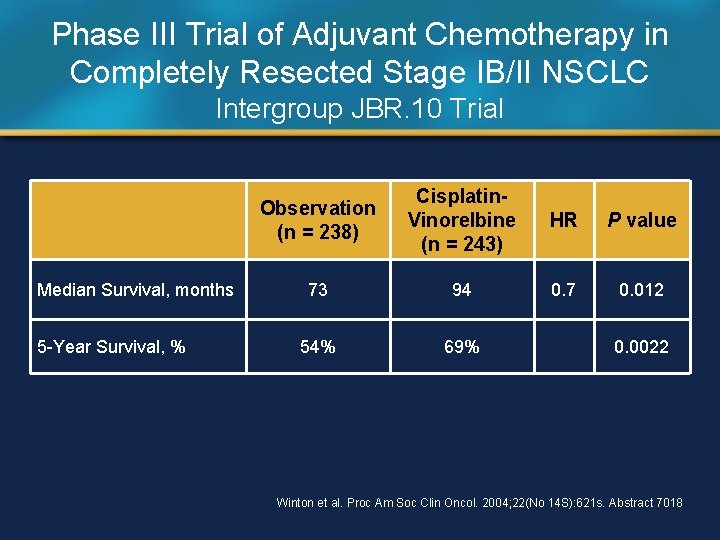
Phase III Trial of Adjuvant Chemotherapy in Completely Resected Stage IB/II NSCLC Intergroup JBR. 10 Trial Median Survival, months 5 -Year Survival, % Observation (n = 238) Cisplatin. Vinorelbine (n = 243) HR P value 73 94 0. 7 0. 012 54% 69% 0. 0022 Winton et al. Proc Am Soc Clin Oncol. 2004; 22(No 14 S): 621 s. Abstract 7018

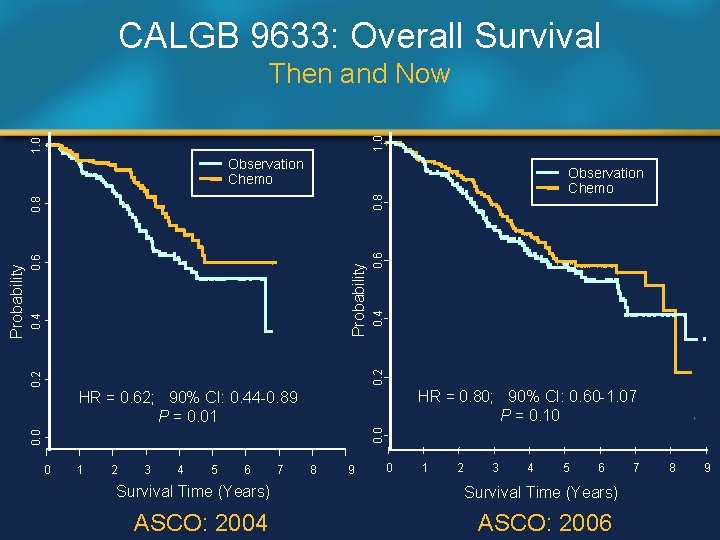
CALGB 9633: Overall Survival 1. 0 Then and Now Observation Chemo 0. 8 0. 6 Probability 0. 2 0. 4 0. 8 0. 6 0. 4 0. 2 HR = 0. 80; 90% CI: 0. 60 -1. 07 P = 0. 10 0. 0 HR = 0. 62; 90% CI: 0. 44 -0. 89 P = 0. 01 0. 0 Probability Observation Chemo 0 1 2 3 4 5 6 Survival Time (Years) ASCO: 2004 7 8 9 0 1 2 3 4 5 6 Survival Time (Years) ASCO: 2006 7 8 9

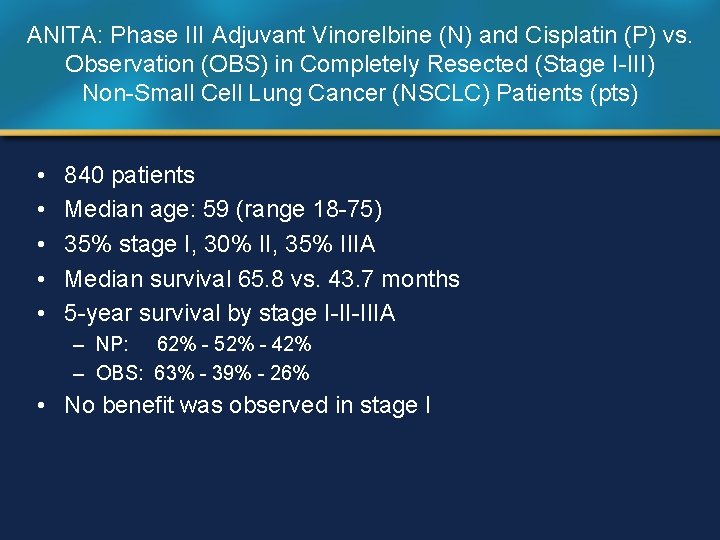
ANITA: Phase III Adjuvant Vinorelbine (N) and Cisplatin (P) vs. Observation (OBS) in Completely Resected (Stage I-III) Non-Small Cell Lung Cancer (NSCLC) Patients (pts) • • • 840 patients Median age: 59 (range 18 -75) 35% stage I, 30% II, 35% IIIA Median survival 65. 8 vs. 43. 7 months 5 -year survival by stage I-II-IIIA – NP: 62% - 52% - 42% – OBS: 63% - 39% - 26% • No benefit was observed in stage I

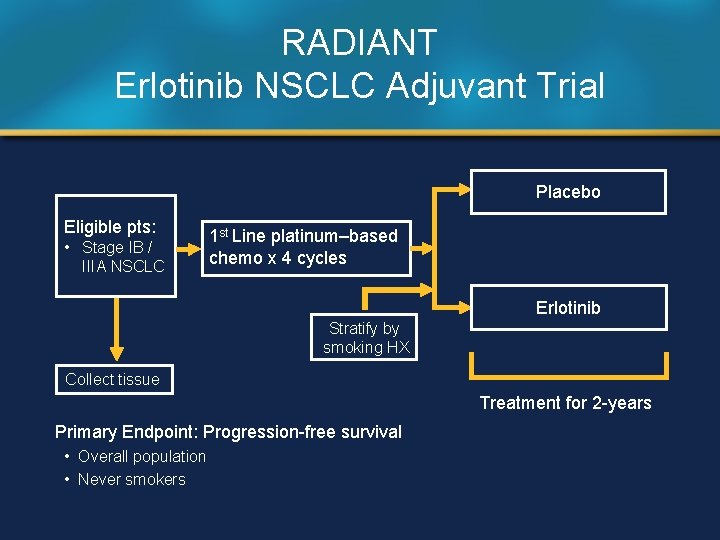
RADIANT Erlotinib NSCLC Adjuvant Trial Placebo Eligible pts: • Stage IB / IIIA NSCLC 1 st Line platinum–based chemo x 4 cycles Erlotinib Stratify by smoking HX Collect tissue Treatment for 2 -years Primary Endpoint: Progression-free survival • Overall population • Never smokers

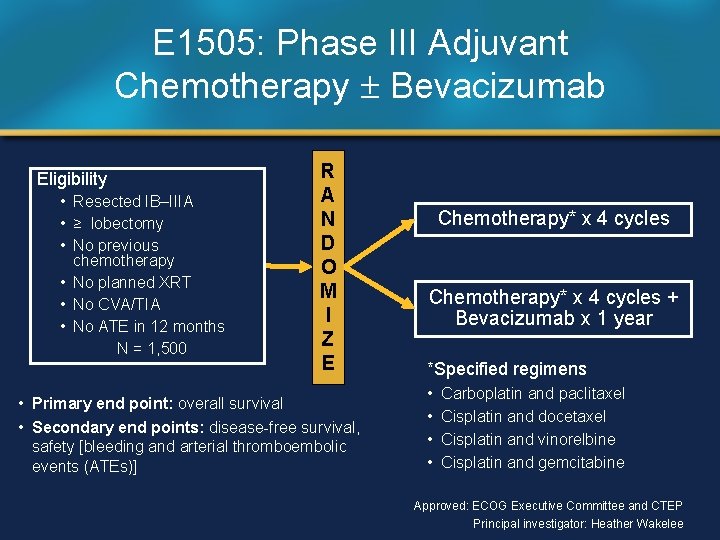
E 1505: Phase III Adjuvant Chemotherapy Bevacizumab Eligibility • Resected IB–IIIA • ≥ lobectomy • No previous chemotherapy • No planned XRT • No CVA/TIA • No ATE in 12 months N = 1, 500 R A N D O M I Z E • Primary end point: overall survival • Secondary end points: disease-free survival, safety [bleeding and arterial thromboembolic events (ATEs)] Chemotherapy* x 4 cycles + Bevacizumab x 1 year *Specified regimens • • Carboplatin and paclitaxel Cisplatin and docetaxel Cisplatin and vinorelbine Cisplatin and gemcitabine Approved: ECOG Executive Committee and CTEP Principal investigator: Heather Wakelee

Case 2: NSCLC • HPI: 69 year old female with h/o T 1 N 1 breast CA x 22 yrs. F/U CXR reveals 3 cm RUL mass. • PMH: HTN, PVD, R Breast CA – 1983 • Meds: Lipitor, Atenolol, Naproxen • Allergies: None • SH: Widow, cigarettes: 50 pack-years • ETOH: none x 1 year • FH: Negative • ROS: Rib pain, unsteady gait, no weight loss • PE: No supraclavicular nodes, C/V: RRR, Resp: clear to A&P, Chest: well healed mastectomy scar, Abd: no masses, Ext: no C/C/E, Neuro: no focal deficits

Case 2: NSCLC • What is the optimal work-up for this patient? 1. 2. 3. 4. Biopsy, CT chest, abdomen, bone scan, MRI brain Referral to medical oncology

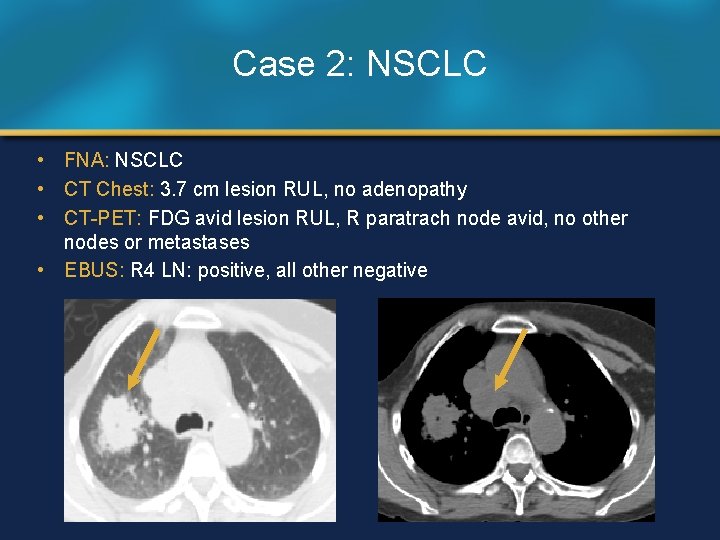
Case 2: NSCLC • FNA: NSCLC • CT Chest: 3. 7 cm lesion RUL, no adenopathy • CT-PET: FDG avid lesion RUL, R paratrach node avid, no other nodes or metastases • EBUS: R 4 LN: positive, all other negative

Case 2: NSCLC • What is the preferred treatment at this time for this patient? 1. 2. 3. 4. Surgery followed by radiation Induction chemotherapy followed by surgery Induction chemoradiotherapy followed by surgery Concurrent chemotherapy and radiation

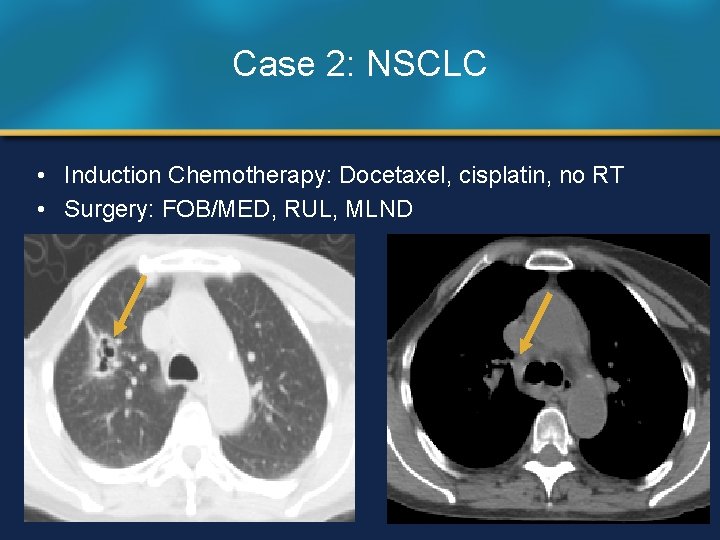
Case 2: NSCLC • Induction Chemotherapy: Docetaxel, cisplatin, no RT • Surgery: FOB/MED, RUL, MLND

Case 2: NSCLC Pathology: T 1 N 2 M 0

Case 2: NSCLC • What is the appropriate next therapy for this patient? 1. 2. 3. 4. Adjuvant chemotherapy followed by radiation Observation Radiation alone

Approach to Resectable Stage IIIA, N 2 NSCLC

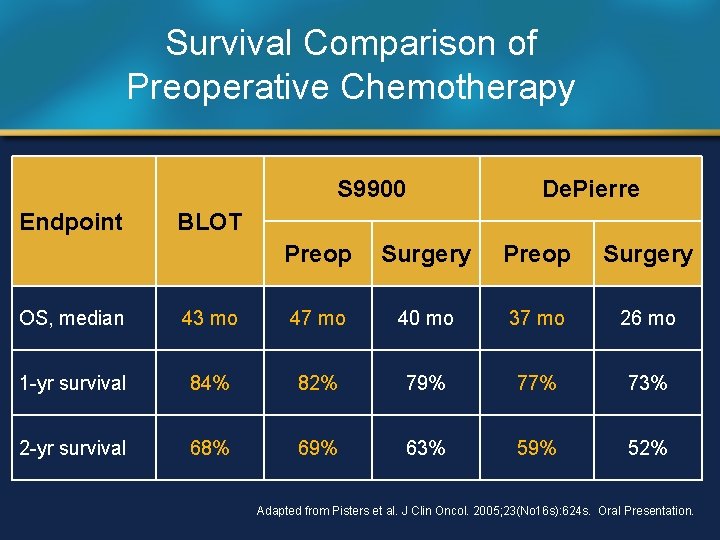
Survival Comparison of Preoperative Chemotherapy S 9900 Endpoint De. Pierre BLOT Preop Surgery OS, median 43 mo 47 mo 40 mo 37 mo 26 mo 1 -yr survival 84% 82% 79% 77% 73% 2 -yr survival 68% 69% 63% 59% 52% Adapted from Pisters et al. J Clin Oncol. 2005; 23(No 16 s): 624 s. Oral Presentation.

Current Issues for Stage IIIA N 2 LN+ Resectable NSCLC • The role of surgery? • Addition of RT to induction chemo increases pathologic CR rates, but also toxicity • Does RT add survival benefit to justify the increased toxicity of bimodality induction therapy for this group of patients? • An ongoing dilemma reflected by variability in treatment approaches across the country • Published clinical trials limited by heterogeneity of patient population(s) studied

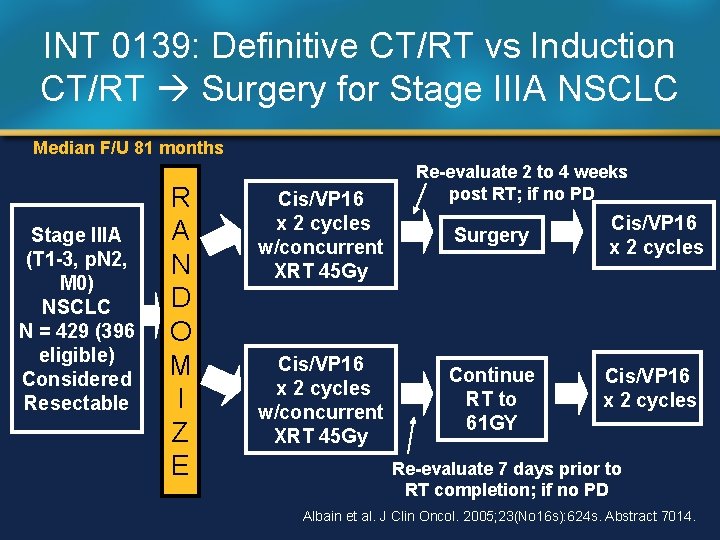
INT 0139: Definitive CT/RT vs Induction CT/RT Surgery for Stage IIIA NSCLC Median F/U 81 months Stage IIIA (T 1 -3, p. N 2, M 0) NSCLC N = 429 (396 eligible) Considered Resectable R A N D O M I Z E Cis/VP 16 x 2 cycles w/concurrent XRT 45 Gy Re-evaluate 2 to 4 weeks post RT; if no PD Surgery Continue RT to 61 GY Cis/VP 16 x 2 cycles Re-evaluate 7 days prior to RT completion; if no PD Albain et al. J Clin Oncol. 2005; 23(No 16 s): 624 s. Abstract 7014.

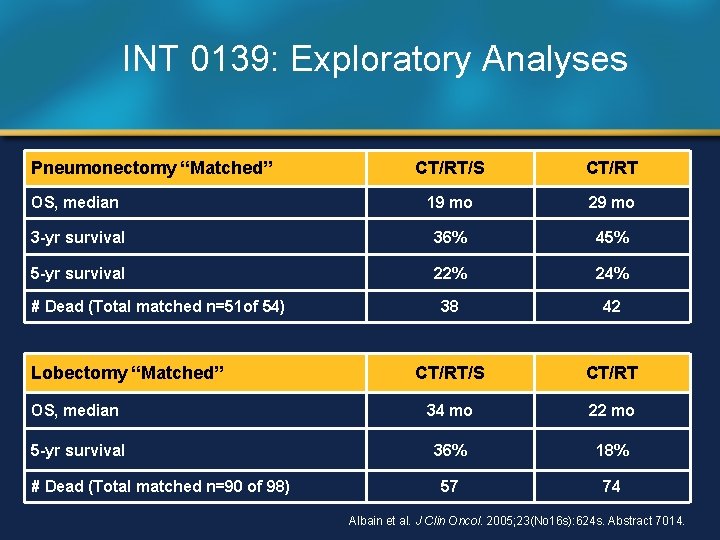
INT 0139: Exploratory Analyses Pneumonectomy “Matched” CT/RT/S CT/RT OS, median 19 mo 29 mo 3 -yr survival 36% 45% 5 -yr survival 22% 24% 38 42 CT/RT/S CT/RT OS, median 34 mo 22 mo 5 -yr survival 36% 18% 57 74 # Dead (Total matched n=51 of 54) Lobectomy “Matched” # Dead (Total matched n=90 of 98) Albain et al. J Clin Oncol. 2005; 23(No 16 s): 624 s. Abstract 7014.

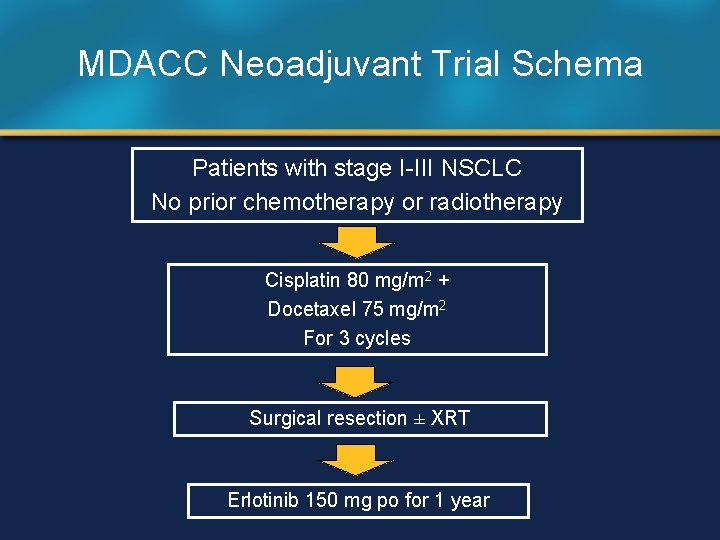
MDACC Neoadjuvant Trial Schema Patients with stage I-III NSCLC No prior chemotherapy or radiotherapy Cisplatin 80 mg/m 2 + Docetaxel 75 mg/m 2 For 3 cycles Surgical resection ± XRT Erlotinib 150 mg po for 1 year

Who’s Appropriate for Multimodality Surgical Resection? • Microscopic single station N 2 • T 4 N 0 -1 • Perhaps responding larger N 2

Stage IIIA “Bulky” N 2 and Stage IIIB NSCLC • Multimodality approach with chemotherapy and radiation therapy – Randomized evidence of survival benefit of chemo-RT over RT alone – Concurrent: generally accepted as standard definitive treatment of patients with good PF – Sequential: less toxic; defendable treatment option • Unclear impact of surgery on local control in combined modality approach – Downstaging

Case 3: NSCLC • HPI: 62 year old male with DJD. C/o 8 -10 months of progressive SOB. Spirometry normal. – Continued SOB for few months, saw pulmonologist. Repeat spirometry, Advair started. CXR revealed 4 cm RML lesion and moderate pleural effusion. • PMH: DJD • Meds: Celebrex prn • Allergies: None • SH: Married, never smoker • ETOH: Rare • FH: Negative • ROS: Rib pain, SOB, no weight loss • PE: No supraclavicular nodes, C/V: RRR, Resp: clear to A&P, Chest: well healed mastectomy scar, Abd: no masses, Ext: no C/C/E, Neuro: no focal deficits

Case 3: NSCLC • What is the optimal work-up at this time? 1. Biopsy, CT chest, abdomen 2. Thoracentesis, CT chest, abdomen, bone scan 3. Thoracentesis, CT chest, abdomen, bone scan, brain scan 4. Hospice care

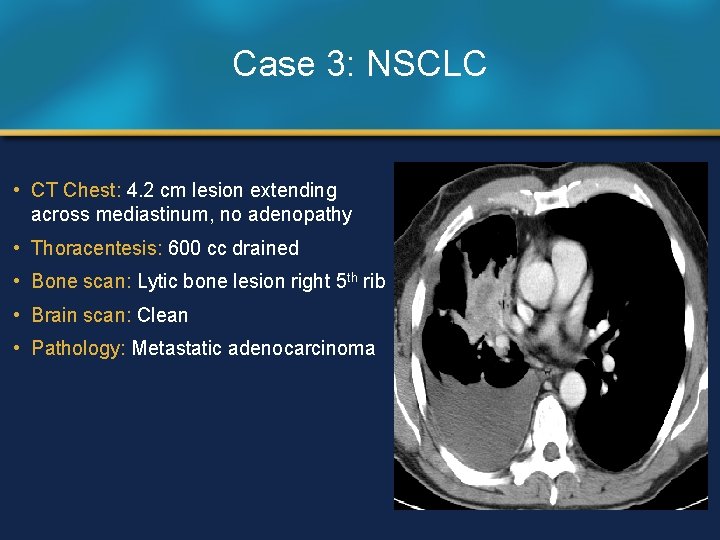
Case 3: NSCLC • CT Chest: 4. 2 cm lesion extending across mediastinum, no adenopathy • Thoracentesis: 600 cc drained • Bone scan: Lytic bone lesion right 5 th rib • Brain scan: Clean • Pathology: Metastatic adenocarcinoma

Case 3: NSCLC • What is the optimal treatment for this patient? 1. 2. 3. 4. 5. 6. Cisplatin doublet Carboplatin + docetaxel Carboplatin + paclitaxel Carboplatin + gemcitabine Carboplatin + taxane + bevacizumab Bevacizumab + erlotinib

Case 3: NSCLC • Standard – Doublet chemotherapy • Standard “plus” – Doublet chemotherapy + bevacizumab • Non-standard – Erlotinib

Case 3: NSCLC • Started: carboplatin + docetaxel + bevacizumab 9 -12 -05 11 -30 -05

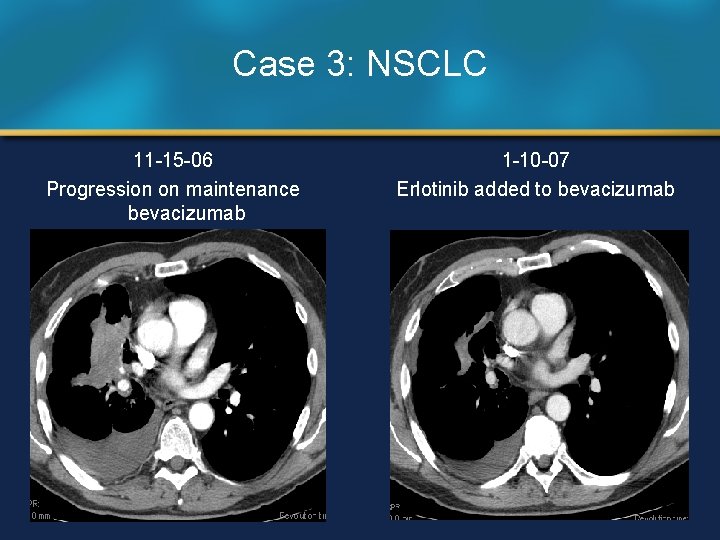
Case 3: NSCLC 11 -15 -06 Progression on maintenance bevacizumab 1 -10 -07 Erlotinib added to bevacizumab

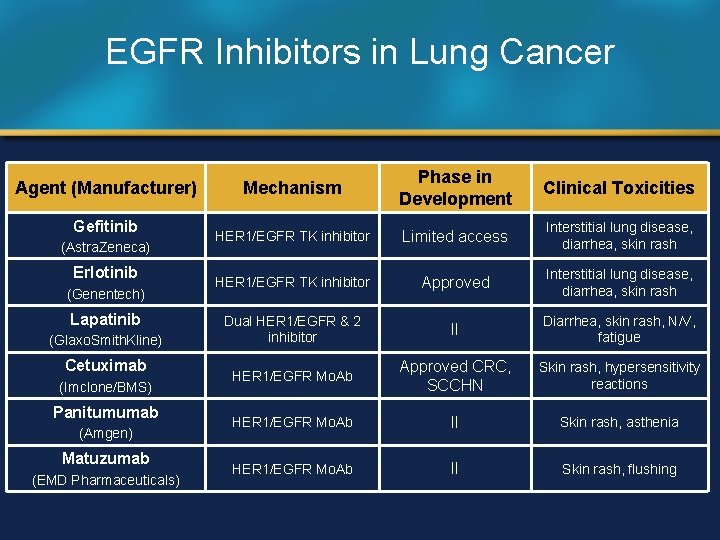
EGFR Inhibitors in Lung Cancer Agent (Manufacturer) Gefitinib (Astra. Zeneca) Erlotinib (Genentech) Lapatinib (Glaxo. Smith. Kline) Cetuximab (Imclone/BMS) Panitumumab (Amgen) Matuzumab (EMD Pharmaceuticals) Mechanism Phase in Development Clinical Toxicities HER 1/EGFR TK inhibitor Limited access Interstitial lung disease, diarrhea, skin rash HER 1/EGFR TK inhibitor Approved Interstitial lung disease, diarrhea, skin rash Dual HER 1/EGFR & 2 inhibitor II Diarrhea, skin rash, N/V, fatigue HER 1/EGFR Mo. Ab Approved CRC, SCCHN Skin rash, hypersensitivity reactions HER 1/EGFR Mo. Ab II Skin rash, asthenia HER 1/EGFR Mo. Ab II Skin rash, flushing

Angiogenesis Inhibitors in Lung Cancer Agent Mechanism Phase in Development Manufacturer Bevacizumab Mo. Ab-Inhibits VEGF binding III Genentech AE-941 Inhibits VEGF binding & MMPs III AEterna Laboratories VEGF trap Blocks VEGF-1 and VEGF-2 I → II Regeneron/ sanofi-aventis Multi-targeted TKIs that includes VEGF inhibition: ZD 6474 VEGFR-2, EGFR II → III Astra. Zeneca ZD 2171 VEGFR-1, VEGFR-2, VEGFR-3 I/II Astra. Zeneca Sunitinib pan-VEGFR, PDGF, RET, others II Pfizer Sorafenib VEGFR-2, PDGF, RAF, others II → III Bayer/Onyx AMG 706 VEGFR, PDGF, Kit, RET II Amgen AEE 788 VEGFR, HER 2 I/II Novartis Vatalanib pan-VEGF inhibitor II Schering/Novartis

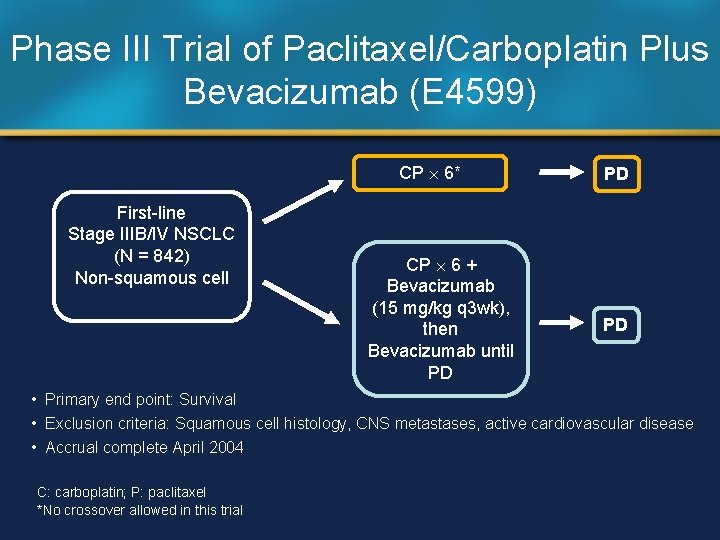
Phase III Trial of Paclitaxel/Carboplatin Plus Bevacizumab (E 4599) CP 6* First-line Stage IIIB/IV NSCLC (N = 842) Non-squamous cell CP 6 + Bevacizumab (15 mg/kg q 3 wk), then Bevacizumab until PD PD PD • Primary end point: Survival • Exclusion criteria: Squamous cell histology, CNS metastases, active cardiovascular disease • Accrual complete April 2004 C: carboplatin; P: paclitaxel *No crossover allowed in this trial

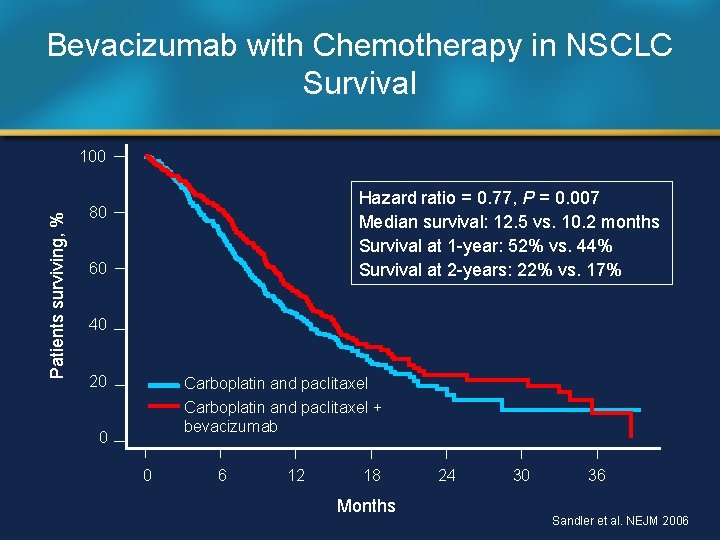
Bevacizumab with Chemotherapy in NSCLC Survival Patients surviving, % 100 Hazard ratio = 0. 77, P = 0. 007 Median survival: 12. 5 vs. 10. 2 months Survival at 1 -year: 52% vs. 44% Survival at 2 -years: 22% vs. 17% 80 60 40 20 Carboplatin and paclitaxel + bevacizumab 0 0 6 12 18 Months 24 30 36 Sandler et al. NEJM 2006

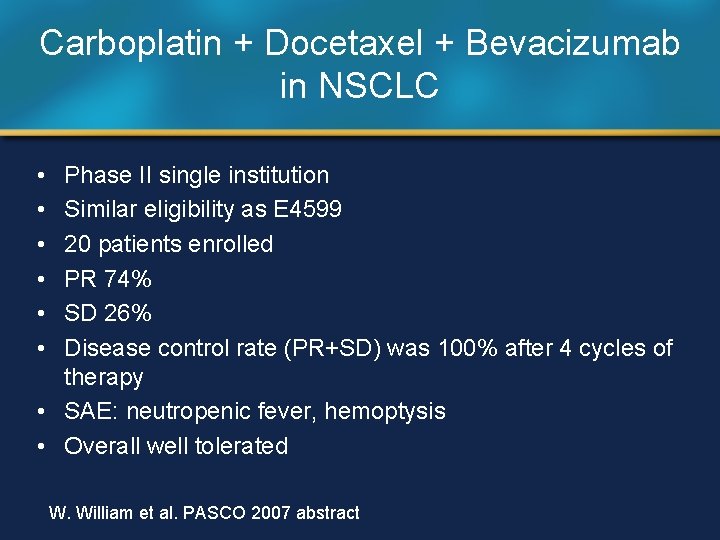
Carboplatin + Docetaxel + Bevacizumab in NSCLC • • • Phase II single institution Similar eligibility as E 4599 20 patients enrolled PR 74% SD 26% Disease control rate (PR+SD) was 100% after 4 cycles of therapy • SAE: neutropenic fever, hemoptysis • Overall well tolerated W. William et al. PASCO 2007 abstract

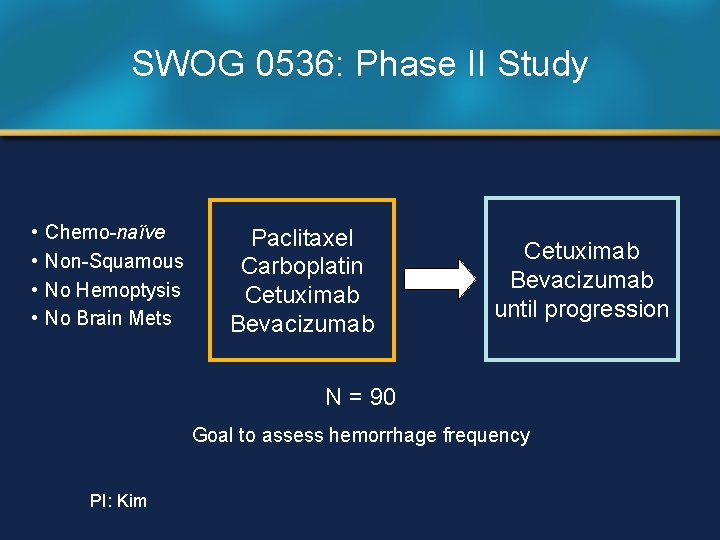
SWOG 0536: Phase II Study • • Chemo-naïve Non-Squamous No Hemoptysis No Brain Mets Paclitaxel Carboplatin Cetuximab Bevacizumab until progression N = 90 Goal to assess hemorrhage frequency PI: Kim

Erlotinib in NSCLC BR. 21: Overall Survival Distribution Function 1. 00 42. 5% improvement in median survival 0. 75 Erlotinib (n = 488) Placebo (n = 243) Median survival (months) 6. 7 4. 7 1 -year survival (%) 31 21 HR = 0. 73, P < 0. 001 0. 50 31% 0. 25 Erlotinib 21% Placebo 0 0 5 10 15 20 25 30 *HR and P-value adjusted for stratification factors at randomization plus HER 1/EGFR status.

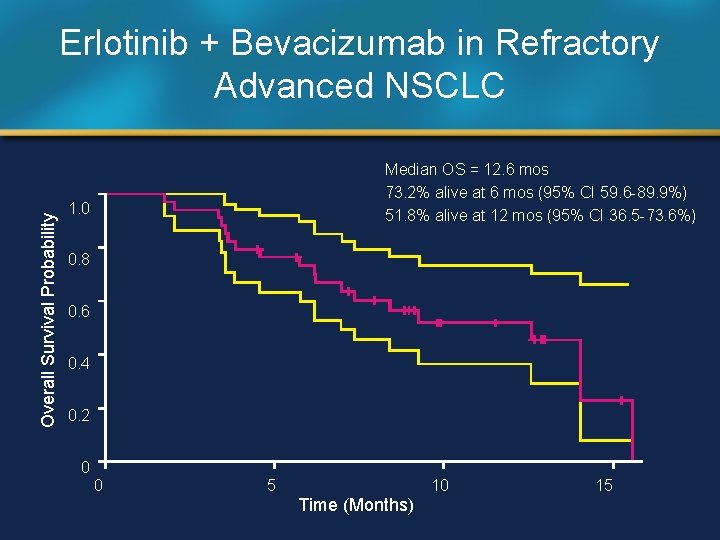
Overall Survival Probability Erlotinib + Bevacizumab in Refractory Advanced NSCLC Median OS = 12. 6 mos 73. 2% alive at 6 mos (95% CI 59. 6 -89. 9%) 51. 8% alive at 12 mos (95% CI 36. 5 -73. 6%) 1. 0 0. 8 0. 6 0. 4 0. 2 0 0 5 10 Time (Months) 15

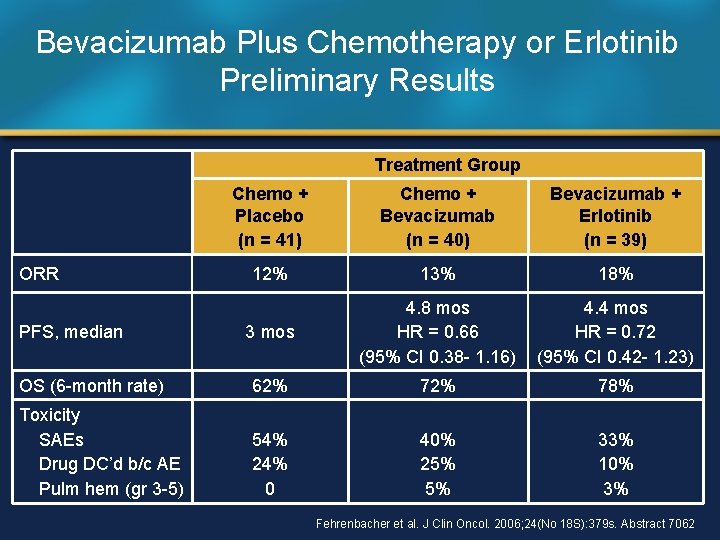
Bevacizumab Plus Chemotherapy or Erlotinib Preliminary Results Treatment Group Chemo + Placebo (n = 41) Chemo + Bevacizumab (n = 40) Bevacizumab + Erlotinib (n = 39) 12% 13% 18% 3 mos 4. 8 mos HR = 0. 66 (95% CI 0. 38 - 1. 16) 4. 4 mos HR = 0. 72 (95% CI 0. 42 - 1. 23) OS (6 -month rate) 62% 78% Toxicity SAEs Drug DC’d b/c AE Pulm hem (gr 3 -5) 54% 24% 0 40% 25% 5% 33% 10% 3% ORR PFS, median Fehrenbacher et al. J Clin Oncol. 2006; 24(No 18 S): 379 s. Abstract 7062

Individualized Therapy • Chemotherapy – Taxanes in breast cancer • Targeted therapy – Trastuzumab in breast – Imitinab in GIST – ? Lung

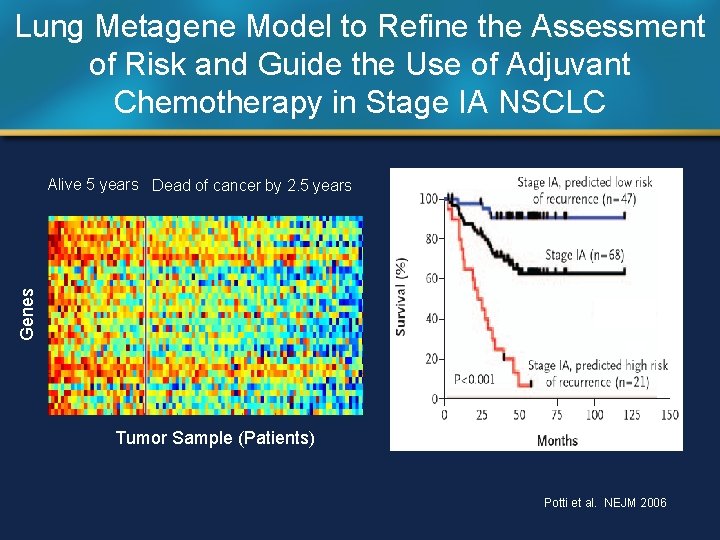
Lung Metagene Model to Refine the Assessment of Risk and Guide the Use of Adjuvant Chemotherapy in Stage IA NSCLC Genes Alive 5 years Dead of cancer by 2. 5 years Tumor Sample (Patients) Potti et al. NEJM 2006

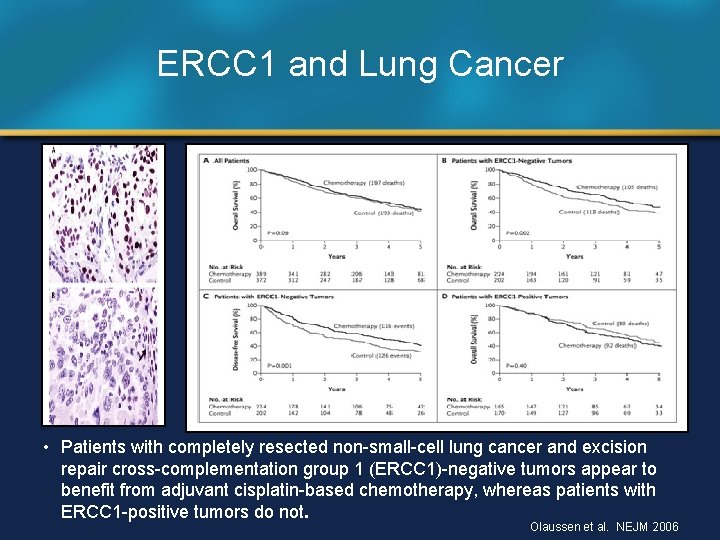
ERCC 1 and Lung Cancer • Patients with completely resected non-small-cell lung cancer and excision repair cross-complementation group 1 (ERCC 1)-negative tumors appear to benefit from adjuvant cisplatin-based chemotherapy, whereas patients with ERCC 1 -positive tumors do not. Olaussen et al. NEJM 2006

BATTLE Biomarker-based Approaches of Targeted Therapy for Lung Cancer Elimination PI: Co-PI: Waun Ki Hong, MD Edward S. Kim, MD Roy Herbst, MD, Ph. D Li Mao, MD

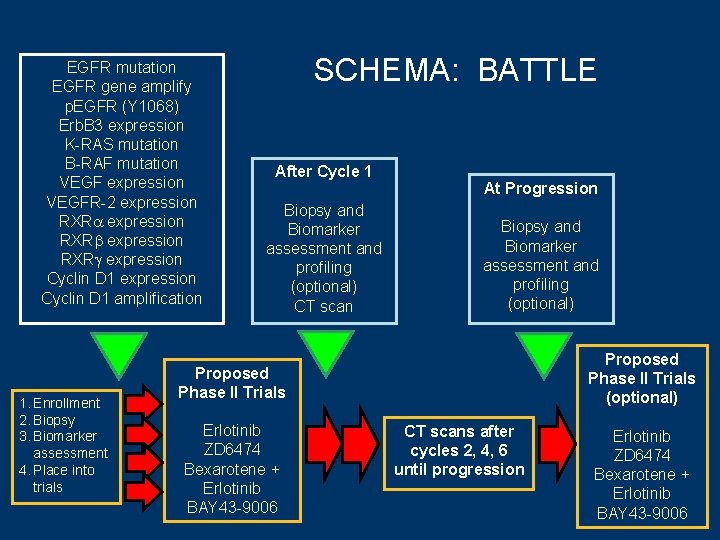
BATTLE Workflow Timeline Registration BIOPSY Response Assign Trial Adaptive Randomization Patient Registration Consent Evaluate Eligibility Baseline Visit Collect Tissue Biomarker Profile Trial Specific Consent Signed Drug Starts Measure Response 8 -weeks 2 -weeks 5 Biomarker Groups EGFR (+) K-RAS(+) or B-RAF(+) VEGF(+) or VEGFR(+) RXRs(+) or Cyclin D 1(+) All(-) Biomarkers used to randomize based on pre-study hypothesis Randomize Treatment

EGFR mutation EGFR gene amplify p. EGFR (Y 1068) Erb. B 3 expression K-RAS mutation B-RAF mutation VEGF expression VEGFR-2 expression RXR expression Cyclin D 1 amplification 1. Enrollment 2. Biopsy 3. Biomarker assessment 4. Place into trials SCHEMA: BATTLE After Cycle 1 Biopsy and Biomarker assessment and profiling (optional) CT scan Proposed Phase II Trials Erlotinib ZD 6474 Bexarotene + Erlotinib BAY 43 -9006 At Progression Biopsy and Biomarker assessment and profiling (optional) CT scans after cycles 2, 4, 6 until progression Proposed Phase II Trials (optional) Erlotinib ZD 6474 Bexarotene + Erlotinib BAY 43 -9006

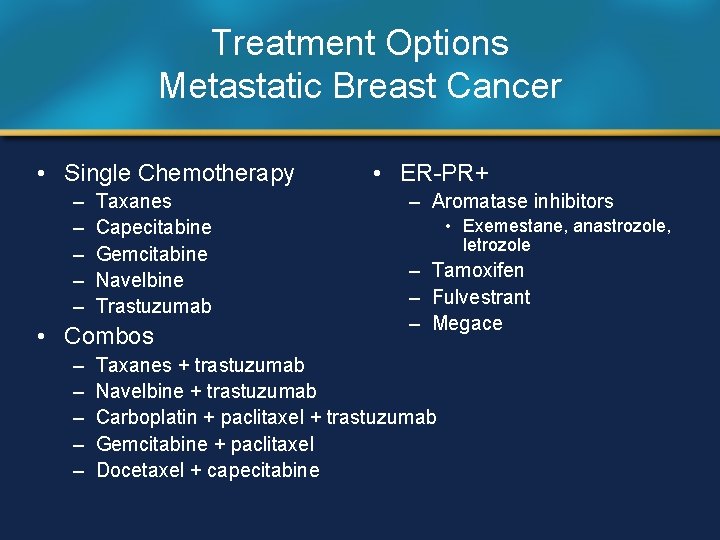
Treatment Options Metastatic Breast Cancer • Single Chemotherapy – – – Taxanes Capecitabine Gemcitabine Navelbine Trastuzumab • Combos – – – • ER-PR+ – Aromatase inhibitors • Exemestane, anastrozole, letrozole – Tamoxifen – Fulvestrant – Megace Taxanes + trastuzumab Navelbine + trastuzumab Carboplatin + paclitaxel + trastuzumab Gemcitabine + paclitaxel Docetaxel + capecitabine

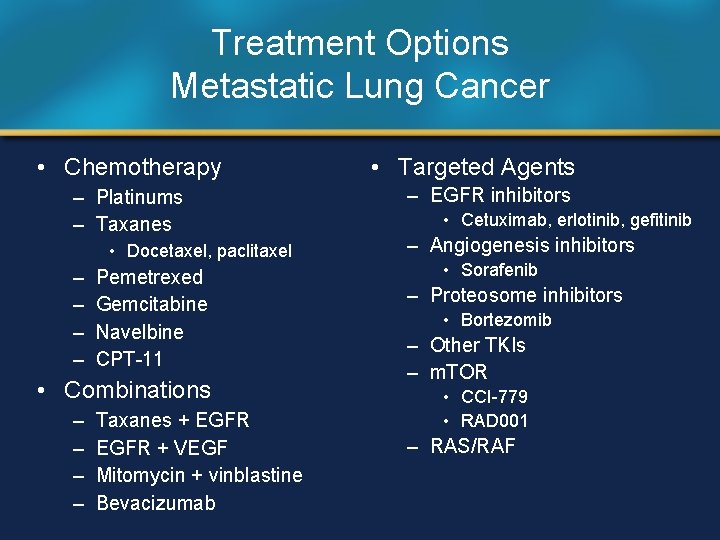
Treatment Options Metastatic Lung Cancer • Chemotherapy – Platinums – Taxanes • Docetaxel, paclitaxel – – Pemetrexed Gemcitabine Navelbine CPT-11 • Combinations – – Taxanes + EGFR + VEGF Mitomycin + vinblastine Bevacizumab • Targeted Agents – EGFR inhibitors • Cetuximab, erlotinib, gefitinib – Angiogenesis inhibitors • Sorafenib – Proteosome inhibitors • Bortezomib – Other TKIs – m. TOR • CCI-779 • RAD 001 – RAS/RAF

Future Objectives • Define a high-risk population – Prior to diagnosis – After curative treatment • Screening strategy • Better patient-defined treatments – Chemotherapy – Targeted therapy

Principles of Lung Cancer Therapy • Chemotherapy and targeted therapy with similar efficacy • Paradigm is in evolution • Targeted ± chemotherapy/targeted combinations investigated • Performance status and quality of life very important to patients • Tissue-based personalized therapy is the future • Looking forward to the next few years
 Nsclc
Nsclc Domenico galetta
Domenico galetta Adjuvant nsclc
Adjuvant nsclc Choking heimlich
Choking heimlich Ktu programming paradigms notes
Ktu programming paradigms notes Distributed systems: principles and paradigms
Distributed systems: principles and paradigms Biological paradigm of psychopathology
Biological paradigm of psychopathology 3 paradigms
3 paradigms Distributed systems principles and paradigms
Distributed systems principles and paradigms Boyfriend/girlfriend centered paradigm
Boyfriend/girlfriend centered paradigm Development support communication
Development support communication Message ordering paradigms
Message ordering paradigms R programming language paradigms
R programming language paradigms Enemy centered paradigm
Enemy centered paradigm The difference between positivism and interpretivism
The difference between positivism and interpretivism Development paradigms
Development paradigms Paradigmatic analysis
Paradigmatic analysis Issues for multimedia authoring
Issues for multimedia authoring Distributed systems principles and paradigms
Distributed systems principles and paradigms Features of php programming language
Features of php programming language Peter hall policy paradigms
Peter hall policy paradigms Binding in programming paradigms
Binding in programming paradigms What are paradigms in hci
What are paradigms in hci Distributed systems principles and paradigms
Distributed systems principles and paradigms Evaluation paradigms
Evaluation paradigms Is a paradigm of distributed computing
Is a paradigm of distributed computing Paradigms and principles
Paradigms and principles Careershodh
Careershodh It infrastructure and emerging technologies
It infrastructure and emerging technologies Jeffrey arnett emerging adulthood theory
Jeffrey arnett emerging adulthood theory Trs ppms pay
Trs ppms pay Site:slidetodoc.com
Site:slidetodoc.com Marketing strategies for emerging markets
Marketing strategies for emerging markets Explain emerging patterns of state politics in india
Explain emerging patterns of state politics in india Swinburne emerging leader
Swinburne emerging leader Emerging sources citation index means
Emerging sources citation index means The byzantine empire and emerging europe answer key
The byzantine empire and emerging europe answer key Atlas of emerging jobs
Atlas of emerging jobs Trish livingston deakin
Trish livingston deakin An emerging world power
An emerging world power It infrastructure and emerging technologies
It infrastructure and emerging technologies Epidemiological triad of malaria
Epidemiological triad of malaria Postformal thought
Postformal thought Challenges of international business in emerging markets
Challenges of international business in emerging markets Emerging trends in disaster mitigation
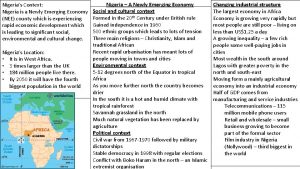
Emerging trends in disaster mitigation Is nigeria a newly emerging economy
Is nigeria a newly emerging economy Memory technology in computer architecture
Memory technology in computer architecture Soft trends in software engineering
Soft trends in software engineering Nacada emerging leaders program
Nacada emerging leaders program Emerging markets inflation
Emerging markets inflation Jeffrey arnett emerging adulthood theory
Jeffrey arnett emerging adulthood theory The hidden risks in emerging markets
The hidden risks in emerging markets Emerging trends in business intelligence
Emerging trends in business intelligence Emerging technology synthesis
Emerging technology synthesis Emerging adulthood psychosocial development
Emerging adulthood psychosocial development Renaissance emerging markets fund
Renaissance emerging markets fund Dubai emerging market
Dubai emerging market Emerging technology chapter 4
Emerging technology chapter 4 It infrastructure and emerging technologies
It infrastructure and emerging technologies Emerging technologies in ict
Emerging technologies in ict Mainframe and minicomputer era
Mainframe and minicomputer era Levinson seasons of life theory
Levinson seasons of life theory Epfr global fund flows
Epfr global fund flows Emerging database technologies and applications
Emerging database technologies and applications World heart federation emerging leaders
World heart federation emerging leaders Chapter 5 it infrastructure and emerging technologies
Chapter 5 it infrastructure and emerging technologies Emerging issues in taxation
Emerging issues in taxation