The PLATINUM DIVERSITY Study Outcomes in Women and









- Slides: 26

The PLATINUM DIVERSITY Study Outcomes in Women and Minorities Compared with White Men One Year After Everolimus-eluting Stent Implantation Wayne Batchelor, M. D. and Roxana Mehran, M. D. on behalf of the PLATINUM DIVERSITY investigators

Financial Disclosures Within the past 12 months, I or my spouse/partner have had a financial interest/arrangement or affiliation with the organization(s) listed below. Affiliation/Financial Relationship Company • Grant/Research Support • Boston Scientific • Consulting Fees/Honoraria • Abbott, Medtronic • Boston Scientific

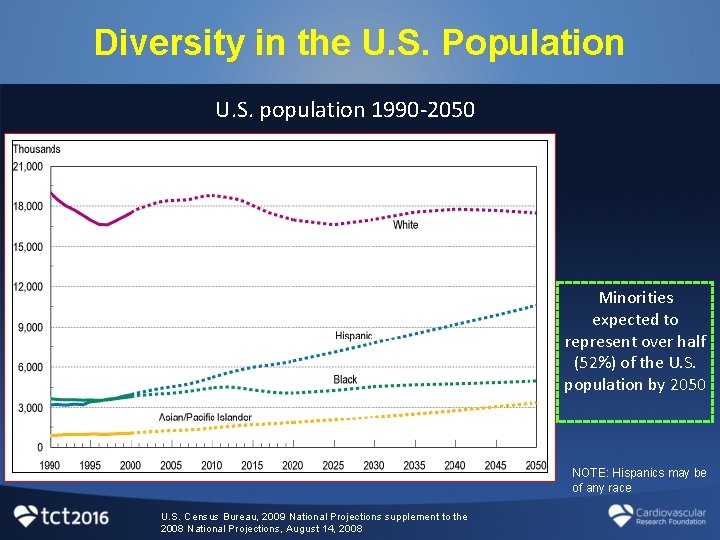
Diversity in the U. S. Population U. S. population 1990 -2050 Minorities expected to represent over half (52%) of the U. S. population by 2050 NOTE: Hispanics may be of any race U. S. Census Bureau, 2009 National Projections supplement to the 2008 National Projections, August 14, 2008

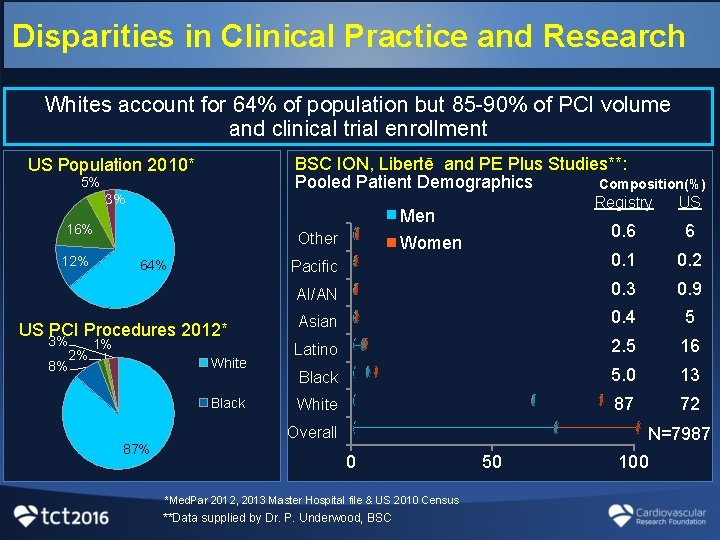
Disparities in Clinical Practice and Research Whites account for 64% of population but 85 -90% of PCI volume and clinical trial enrollment BSC ION, Libertē and PE Plus Studies**: Pooled Patient Demographics Composition(%) US Population 2010* 5% 3% US 0. 6 6 Pacific 0. 1 0. 2 AI/AN 0. 3 0. 9 Asian 0. 4 5 Latino 2. 5 16 Black 5. 0 13 White 87 72 Men 16% 12% Registry Other 64% US PCI Procedures 2012* 3% 1% 2% 8% White Black Women Overall 87% N=7987 0 *Med. Par 2012, 2013 Master Hospital file & US 2010 Census **Data supplied by Dr. P. Underwood, BSC 50 100

PURPOSE 1. Construct a novel study design that would specifically address the diversity gap 2. Determine if there are significant differences in 1 year clinical outcomes between women and minorities compared with white men in the era of second generation DES 3. To characterize and evaluate the impact of social/behavioral/economic determinants of health in women and minorities

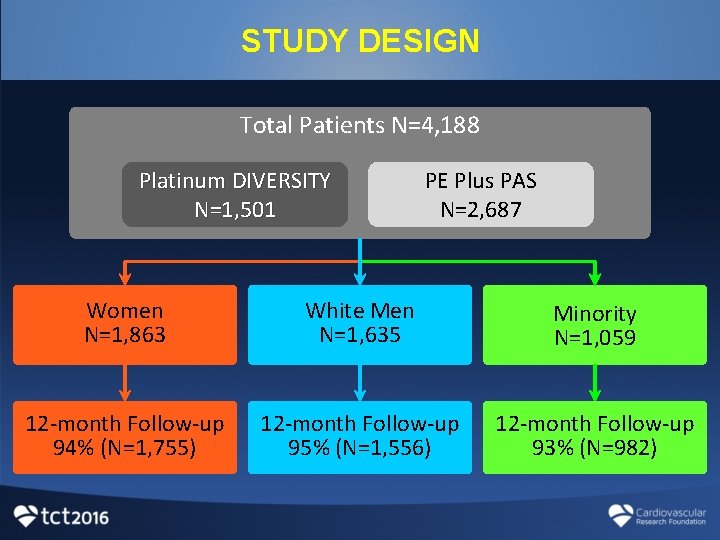
PLATINUM DIVERSITY STUDY DESIGN • Multicenter, single-arm, prospective “enriched” cohort study • 52 U. S. sites enrolling women and minorities • Pooling of data with PROMUS ELEMENT PAS f (I) PLATINUM DIVERSITY COHORT (n=1, 501) Inclusion criteria • Patients received at least 1 Promus PREMIER stent • Self-identified as ≥ 1 of the following: - Female - Black, of African heritage - Hispanic/Latino - American Indian/Alaskan Native (II) PROMUS ELEMENT PLUS PAS COHORT (n=2, 687)* • prospective, open-label, multicenter, all-comers observational study designed to examine 1 yr outcomes with PROMUS Element Plus stent *Kandzari et al Am J Cardiol. 2016

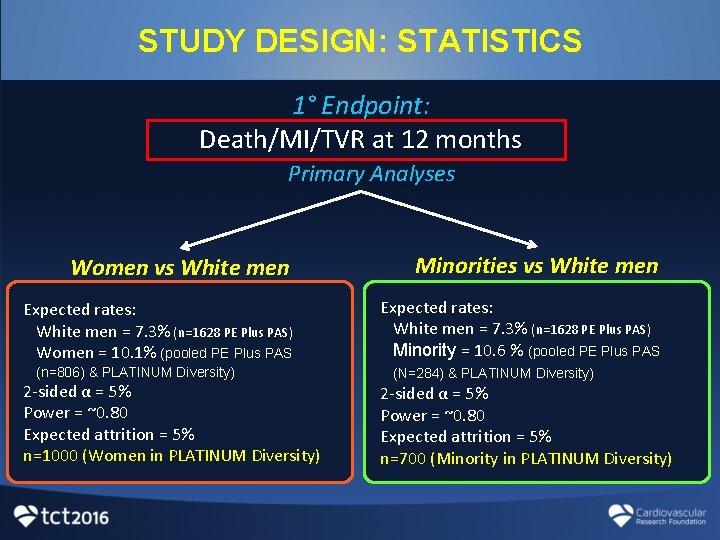
STUDY DESIGN: STATISTICS 1° Endpoint: Death/MI/TVR at 12 months Primary Analyses Women vs White men Minorities vs White men Expected rates: White men = 7. 3% (n=1628 PE Plus PAS) Women = 10. 1% (pooled PE Plus PAS Expected rates: White men = 7. 3% (n=1628 PE Plus PAS) Minority = 10. 6 % (pooled PE Plus PAS (n=806) & PLATINUM Diversity) 2 -sided α = 5% Power = ~0. 80 Expected attrition = 5% n=1000 (Women in PLATINUM Diversity) (N=284) & PLATINUM Diversity) 2 -sided α = 5% Power = ~0. 80 Expected attrition = 5% n=700 (Minority in PLATINUM Diversity)

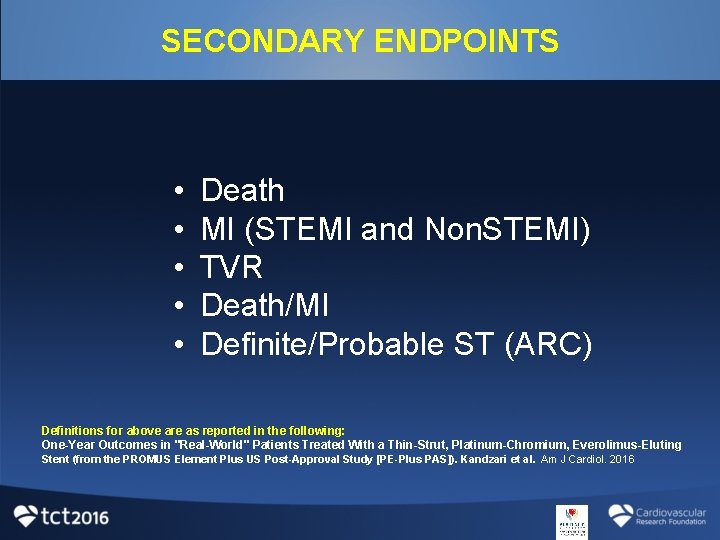
SECONDARY ENDPOINTS • • • Death MI (STEMI and Non. STEMI) TVR Death/MI Definite/Probable ST (ARC) Definitions for above are as reported in the following: One-Year Outcomes in "Real-World" Patients Treated With a Thin-Strut, Platinum-Chromium, Everolimus-Eluting Stent (from the PROMUS Element Plus US Post-Approval Study [PE-Plus PAS]). Kandzari et al. Am J Cardiol. 2016

STUDY DESIGN Total Patients N=4, 188 Platinum DIVERSITY N=1, 501 PE Plus PAS N=2, 687 Women N=1, 863 White Men N=1, 635 Minority N=1, 059 12 -month Follow-up 94% (N=1, 755) 12 -month Follow-up 95% (N=1, 556) 12 -month Follow-up 93% (N=982)

STUDY COMMITTEES Principal Investigators Roxana Mehran Wayne Batchelor Independent Clinical Events Committee Joseph Kannam (chair) Germano Di. Sciascio Claude Hanet Goran Stankovic Study Sponsor CRO Mount Sinai Medical Center Tallahassee Memorial Hospital Boston Scientific Corporation Lisa Currier, Project Manager Project Management, Statistics PRA International Colleen Mullenix, Project Manager Monitoring, Data Management, Safety, Statistics

PLATINUM DIVERSITY TOP SITES Study Principal Roxana Mehran (81) Mount Sinai Medical Center Investigators Scott Davis (101) Arkansas Cardiology Luis Tami (96) Research Physicians Network Alliance John Wang (71) Top Enrolling Centers Principal Investigators Med. Star Union Memorial Hospital Islam Othman (69) NC Heart and Vascular Research Osvaldo Gigliotti (65) Seton Heart Institute Amir Haghighat (47) Cardiovascular Institute of Northwest Florida Sarabjeet Singh (46) Central Cardiology Medical Clinic Mario Lopez (43) Charlotte Heart and Vascular Institute Gregory Giugliano (40) Baystate Medical Center Phillip Horwitz (39) University of Iowa Hospitals and Clinics Sandeep Nathan (38) University of Chicago Medical Center Wayne Batchelor (48) Tallahassee Memorial Hospital Roger Hill (38) St Bernards Heart and Vascular Steven Hearne (36) Delmarva Heart Research Foundation Vince Vismara (35) Palmetto Health Jerrold Grodin (34) VA North Texas Health Care System Jerome E Williams (34) Novant Health Heart and Vascular Institute Robert Pyo (33) Montefiore Medical Center Elie Gharib (32) CAMC Clinical Trials Center Hosbedar Tamboli (31) Bay Area Cardiology Zafir Hawa (31) North Kansas City Hospital Tim Shapiro (30) Lankenau Medical Center George Kichura (29) St. Louis Heart and Vascular

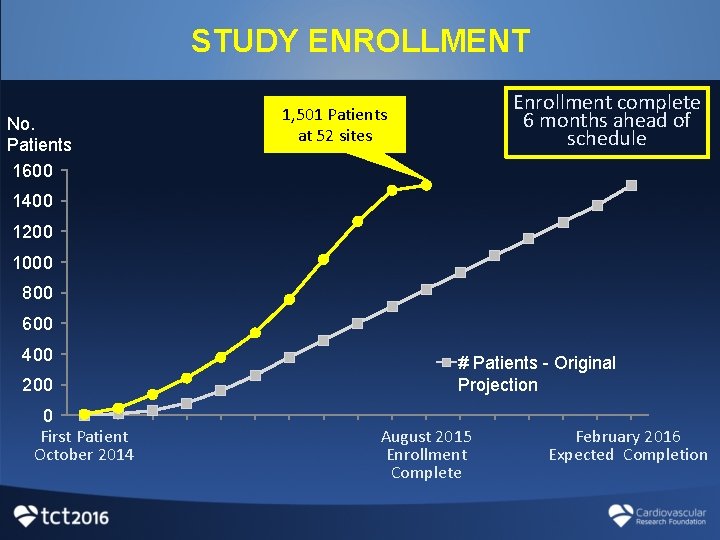
STUDY ENROLLMENT No. Patients 1600 Enrollment complete 6 months ahead of schedule 1, 501 Patients at 52 sites 1400 1200 1000 800 600 400 200 0 First Patient October 2014 # Patients - Original Projection August 2015 Enrollment Complete February 2016 Expected Completion

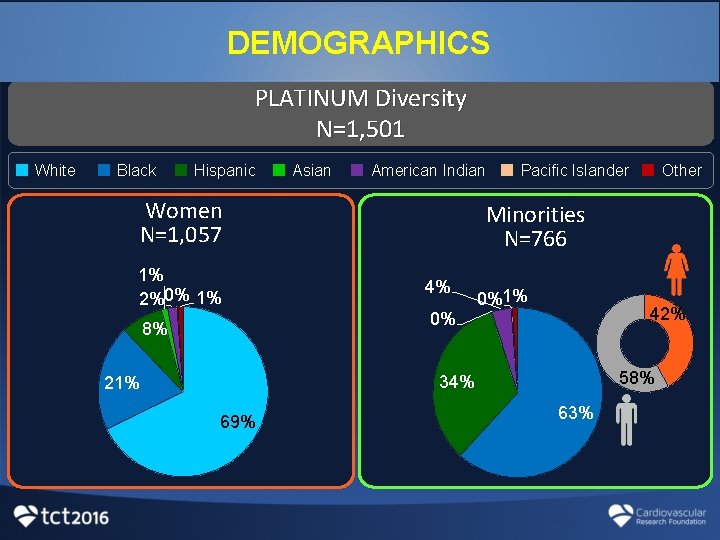
DEMOGRAPHICS PLATINUM Diversity N=1, 501 White Black Hispanic Asian American Indian Women N=1, 057 1% 2%0% 1% 8% Pacific Islander Minorities N=766 4% 0% 0%1% 42% 58% 34% 21% 69% Other 63%

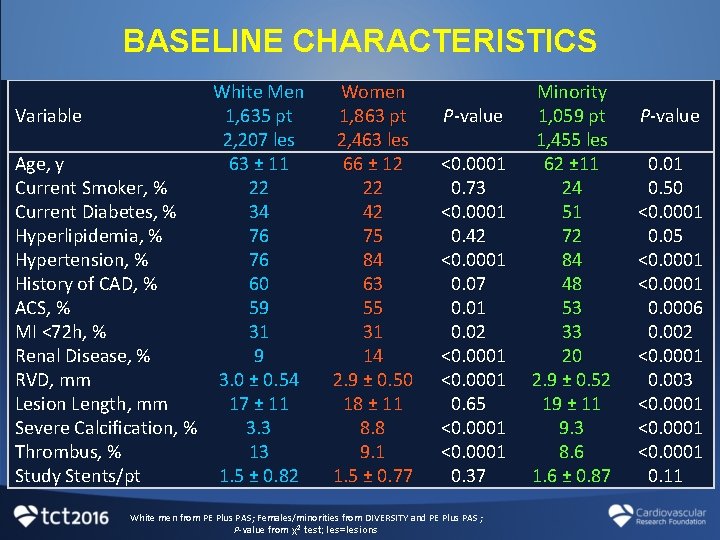
BASELINE CHARACTERISTICS White Men Variable 1, 635 pt 2, 207 les Age, y 63 ± 11 Current Smoker, % 22 Current Diabetes, % 34 Hyperlipidemia, % 76 Hypertension, % 76 History of CAD, % 60 ACS, % 59 MI <72 h, % 31 Renal Disease, % 9 RVD, mm 3. 0 ± 0. 54 Lesion Length, mm 17 ± 11 Severe Calcification, % 3. 3 Thrombus, % 13 Study Stents/pt 1. 5 ± 0. 82 Women 1, 863 pt 2, 463 les 66 ± 12 22 42 75 84 63 55 31 14 2. 9 ± 0. 50 18 ± 11 8. 8 9. 1 1. 5 ± 0. 77 P-value <0. 0001 0. 73 <0. 0001 0. 42 <0. 0001 0. 07 0. 01 0. 02 <0. 0001 0. 65 <0. 0001 0. 37 White men from PE Plus PAS; Females/minorities from DIVERSITY and PE Plus PAS ; P-value from χ2 test; les=lesions Minority 1, 059 pt 1, 455 les 62 ± 11 24 51 72 84 48 53 33 20 2. 9 ± 0. 52 19 ± 11 9. 3 8. 6 1. 6 ± 0. 87 P-value 0. 01 0. 50 <0. 0001 0. 05 <0. 0001 0. 0006 0. 002 <0. 0001 0. 003 <0. 0001 0. 11

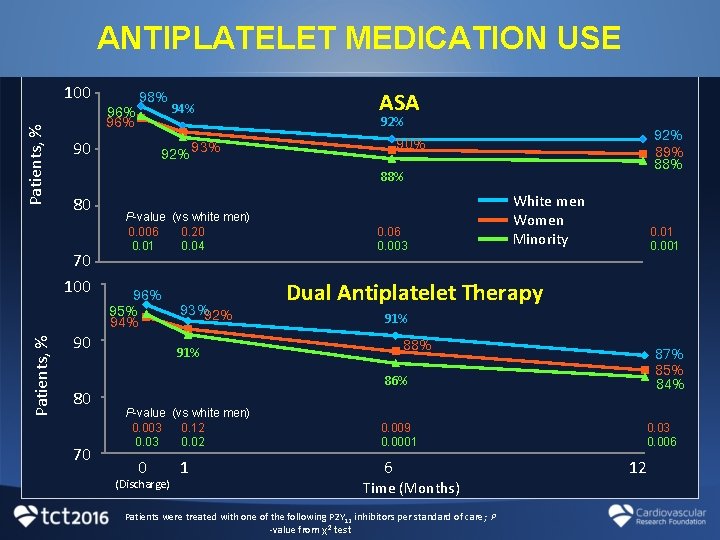
ANTIPLATELET MEDICATION USE Patients, % 100 94% ASA 92% 89% 88% 90% 92% 93% 90 88% 80 70 100 Patients, % 96% 98% 90 80 70 P-value (vs white men) 0. 006 0. 20 0. 01 0. 04 96% 95% 94% 93%92% 0. 06 0. 003 White men Women Minority 0. 01 0. 001 Dual Antiplatelet Therapy 91% 88% 91% 87% 85% 84% 86% P-value (vs white men) 0. 003 0. 12 0. 03 0. 02 0 (Discharge) 1 0. 009 0. 0001 6 Time (Months) Patients were treated with one of the following P 2 Y 12 inhibitors per standard of care; P -value from χ2 test 0. 03 0. 006 12

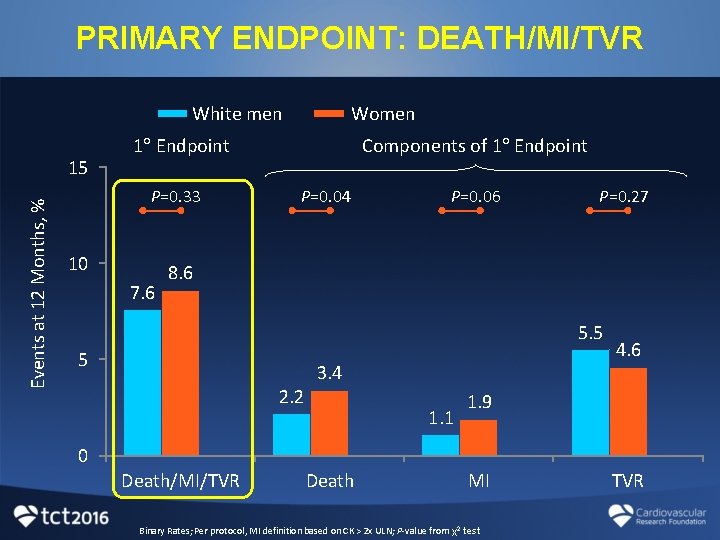
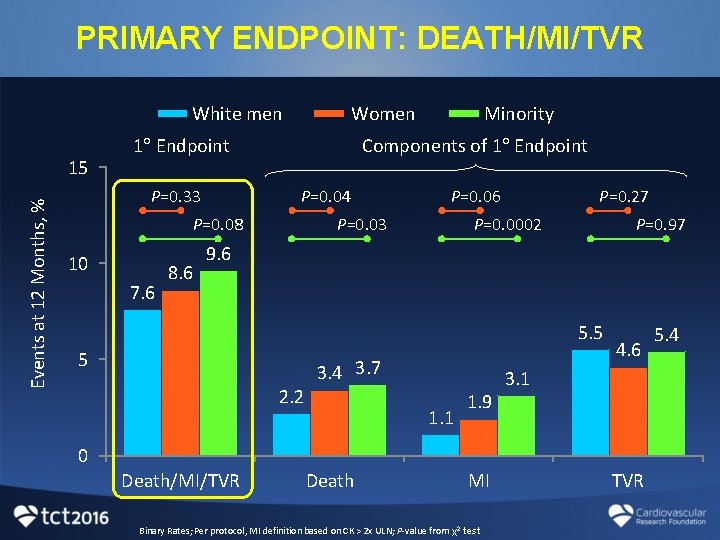
PRIMARY ENDPOINT: DEATH/MI/TVR White men Events at 12 Months, % 15 Women 1 Endpoint P=0. 33 10 7. 6 Components of 1 Endpoint P=0. 04 P=0. 06 P=0. 27 8. 6 5. 5 5 2. 2 3. 4 1. 1 4. 6 1. 9 0 Death/MI/TVR Death MI Binary Rates; Per protocol, MI definition based on CK > 2 x ULN; P-value from χ2 test TVR

PRIMARY ENDPOINT: DEATH/MI/TVR White men Events at 12 Months, % 15 Women 1 Endpoint P=0. 33 Components of 1 Endpoint P=0. 04 P=0. 08 10 7. 6 8. 6 Minority P=0. 06 P=0. 03 P=0. 27 P=0. 0002 P=0. 97 9. 6 5. 5 5 2. 2 3. 4 3. 7 1. 1 1. 9 4. 6 3. 1 0 Death/MI/TVR Death MI Binary Rates; Per protocol, MI definition based on CK > 2 x ULN; P-value from χ2 test TVR 5. 4

SECONDARY ENDPOINT: DEATH/MI Death/MI, % White men Women Minority 10 P-values White men vs Women P=0. 006 8 White men vs Minority P=0. 0002 6. 5 6 5. 1 4 3. 0 2 0 0 180 Time (Days) 365 At-risk Patients White Men 1635 1569 1068 Women 1863 1795 1251 Minority 1059 1017 715 Kaplan Meier Rates; P-value from log-rank test

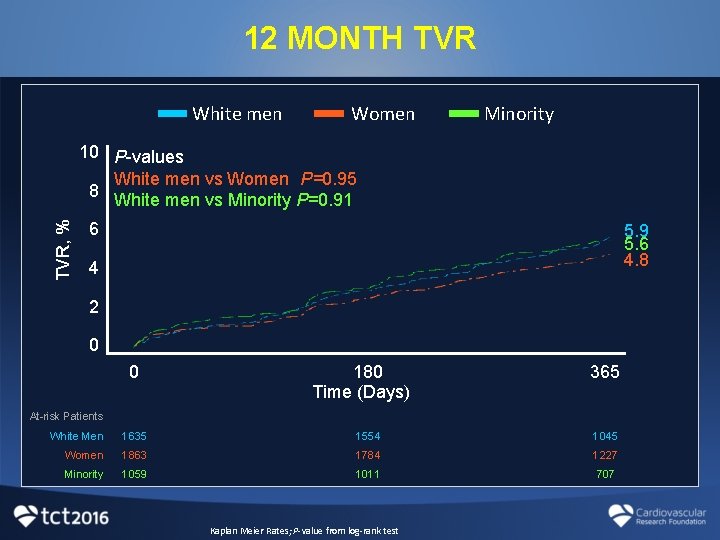
12 MONTH TVR White men Women Minority TVR, % 10 P-values White men vs Women P=0. 95 8 White men vs Minority P=0. 91 6 5. 9 5. 6 4. 8 4 2 0 0 180 Time (Days) 365 At-risk Patients White Men 1635 1554 1045 Women 1863 1784 1227 Minority 1059 1011 707 Kaplan Meier Rates; P-value from log-rank test

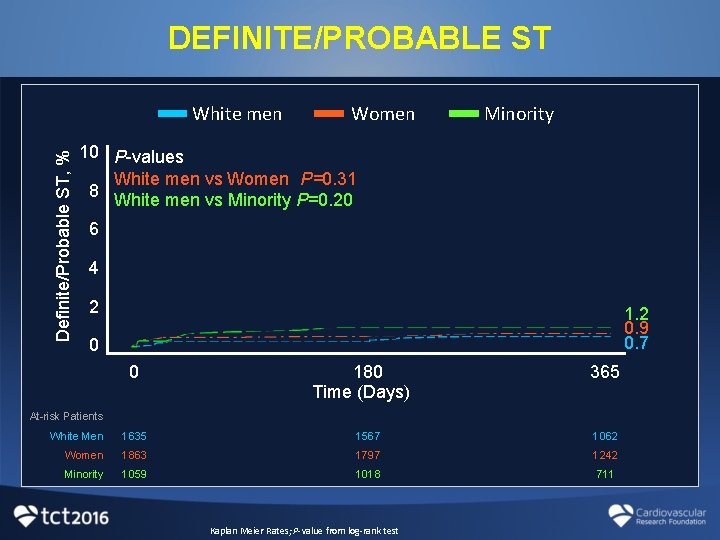
DEFINITE/PROBABLE ST Definite/Probable ST, % White men Women Minority 10 P-values White men vs Women P=0. 31 8 White men vs Minority P=0. 20 6 4 2 1. 2 0. 9 0. 7 0 0 180 Time (Days) 365 At-risk Patients White Men 1635 1567 1062 Women 1863 1797 1242 Minority 1059 1018 711 Kaplan Meier Rates; P-value from log-rank test

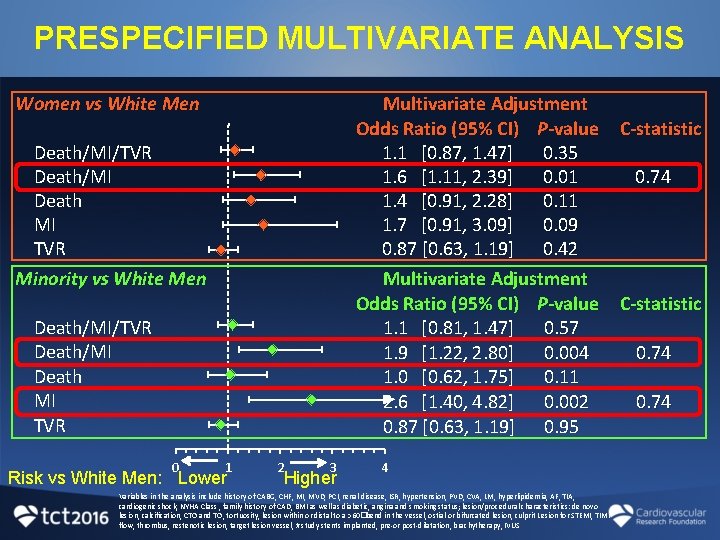
PRESPECIFIED MULTIVARIATE ANALYSIS Women vs White Men Multivariate Adjustment Odds Ratio (95% CI) P-value 1. 1 [0. 87, 1. 47] 0. 35 1. 6 [1. 11, 2. 39] 0. 01 1. 4 [0. 91, 2. 28] 0. 11 1. 7 [0. 91, 3. 09] 0. 09 0. 87 [0. 63, 1. 19] 0. 42 Multivariate Adjustment Odds Ratio (95% CI) P-value 1. 1 [0. 81, 1. 47] 0. 57 1. 9 [1. 22, 2. 80] 0. 004 1. 0 [0. 62, 1. 75] 0. 11 2. 6 [1. 40, 4. 82] 0. 002 0. 87 [0. 63, 1. 19] 0. 95 Death/MI/TVR Death/MI Death MI TVR Minority vs White Men Death/MI/TVR Death/MI Death MI TVR 0 1 Risk vs White Men: Lower 2 3 Higher 4 Variables in the analysis include history of CABG, CHF, MI, MVD, PCI, renal disease, ISR, hypertension, PVD, CVA, LM, hyperlipidemia, AF, TIA, cardiogenic shock, NYHA Class , family history of CAD, BMI as well as diabetic, angina and smoking status; lesion/procedural characteristics: de novo lesion, calcification, CTO and TO, tortuosity, lesion within or distal to a >60�bend in the vessel, ostial or bifurcated lesion, culprit Lesion for STEMI, TIMI flow, thrombus, restenotic lesion, target lesion vessel, # study stents implanted, pre-or post-dilatation, brachytherapy, IVUS C-statistic 0. 74

CONCLUSIONS • “All-comers” coronary stent trials do not represent a random selection of U. S. patients and tend to lack adequate enrollment of women and minorities • By employing an “enriched enrollment” prospective cohort design, PLATINUM DIVERSITY efficiently produced valuable outcomes data to help bridge this gap • Despite significant differences in baseline clinical and angiographic characteristics, women, minorities and white men showed no differences in death/MI/TVR

CONCLUSIONS • Women showed an increased risk of death/MI • Minorities showed an increased risk of MI and death/MI • Similar rates of TVR and ST among all 3 groups suggest that “device failure” is unlikely to account for the observed differences • These results highlight the heterogeneity conferred by sex and race and suggest further study into the biologic, social, behavioral, and economic factors that impact CV risk after DES

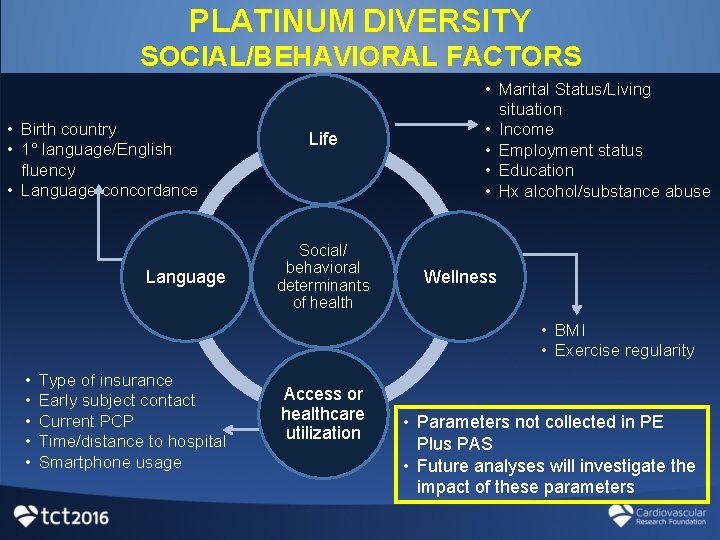
PLATINUM DIVERSITY SOCIAL/BEHAVIORAL FACTORS • Birth country • 1 language/English fluency • Language concordance Language Life Social/ behavioral determinants of health • Marital Status/Living situation • Income • Employment status • Education • Hx alcohol/substance abuse Wellness • BMI • Exercise regularity • • • Type of insurance Early subject contact Current PCP Time/distance to hospital Smartphone usage Access or healthcare utilization • Parameters not collected in PE Plus PAS • Future analyses will investigate the impact of these parameters

ACKNOWLEDGEMENTS • Paul Underwood Dominic Allocco • Peter Mauer Craig Thompson • Eileen Rose Kristine Roy • Songtao Jiang Peter Lang • Eileen Rose Alison Osattin • Peter Lam

End
 Learning objectives for diversity and inclusion training
Learning objectives for diversity and inclusion training Why is genetic diversity important
Why is genetic diversity important Genetic diversity vs species diversity
Genetic diversity vs species diversity Diabetes prevention program outcomes study
Diabetes prevention program outcomes study What is the name of this isotope?
What is the name of this isotope? In a grey backyard she stood
In a grey backyard she stood Chapter 8 human resources culture and diversity
Chapter 8 human resources culture and diversity Chapter 8 human resources culture and diversity
Chapter 8 human resources culture and diversity Chapter 21 temperature heat and expansion
Chapter 21 temperature heat and expansion Platinum point owners association
Platinum point owners association K raheja platinum
K raheja platinum Amway business modeler
Amway business modeler Platinum age of comics
Platinum age of comics Principalplatinum
Principalplatinum Brb millenium platinum
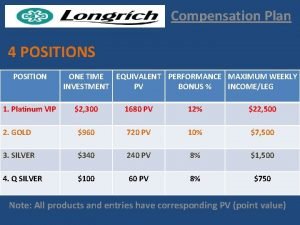
Brb millenium platinum Platinum vip plan
Platinum vip plan Doterra comp plan
Doterra comp plan Beta decay of platinum-199
Beta decay of platinum-199 Pustekkom
Pustekkom Its platinum loops shrink to a wedding-ring
Its platinum loops shrink to a wedding-ring Its platinum loops shrink to a wedding-ring
Its platinum loops shrink to a wedding-ring Platinum 10 minutes ems
Platinum 10 minutes ems Customer pyramid platinum gold iron lead
Customer pyramid platinum gold iron lead Prague lap dancing
Prague lap dancing Platinum periodic table reference
Platinum periodic table reference Glebe harbor
Glebe harbor Platinum periodic table reference
Platinum periodic table reference