The Patient Triage Concept Right Diagnosis Right Treatment







- Slides: 14

The Patient Triage Concept: Right Diagnosis, Right Treatment Susan van den Hof Liverpool 26 October 2016

Conflict of interest disclosure ü I have no, real or perceived, direct or indirect conflicts of interest that relate to this presentation. q I have the following, real or perceived direct or indirect conflicts of interest that relate to this presentation: Affiliation / financial interest Nature of conflict / commercial company name Tobacco-industry and tobacco corporate affiliate related conflict of interest Grants/research support (to myself, my institution or department): Honoraria or consultation fees: Participation in a company sponsored bureau: Stock shareholder: Spouse/partner: Other support or other potential conflict of interest: This event is accredited for CME credits by EBAP and speakers are required to disclose their potential conflict of interest going back 3 years prior to this presentation. The intent of this disclosure is not to prevent a speaker with a conflict of interest (any significant financial relationship a speaker has with manufacturers or providers of any commercial products or services relevant to the talk) from making a presentation, but rather to provide listeners with information on which they can make their own judgment. It remains for audience members to determine whether the speaker’s interests or relationships may influence the presentation. Drug or device advertisement is strictly forbidden.

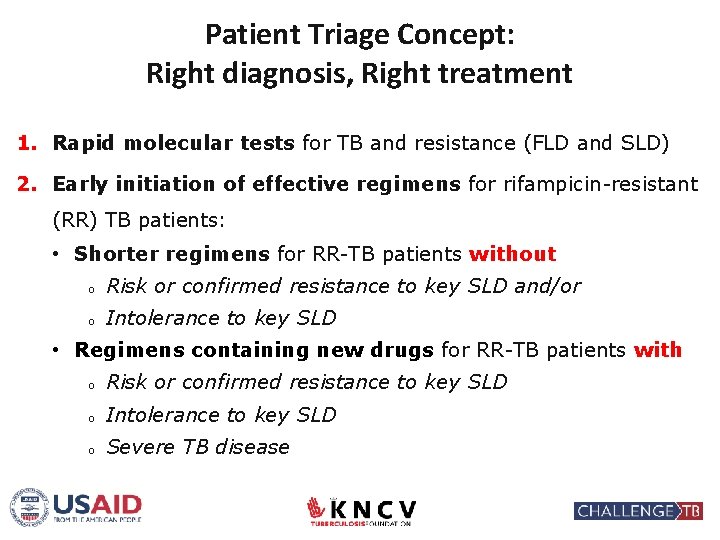
Patient Triage Concept: Right diagnosis, Right treatment 1. Rapid molecular tests for TB and resistance (FLD and SLD) 2. Early initiation of effective regimens for rifampicin-resistant (RR) TB patients: • Shorter regimens for RR-TB patients without o Risk or confirmed resistance to key SLD and/or o Intolerance to key SLD • Regimens containing new drugs for RR-TB patients with o Risk or confirmed resistance to key SLD o Intolerance to key SLD o Severe TB disease

2016 WHO guidance underpins the Patient Triage Concept • Use of molecular line probe assays for detection of second-line drug resistance • Treatment guidelines for DR-TB Shorter DR-TB treatment regimen (STR) recommended under eligibility criteria n Design of individualized treatment regimen uses different grouping of component medicines n

Patient Triage Concept Linking the bedaquiline donation program with introduction of the shorter DR-TB treatment regimen

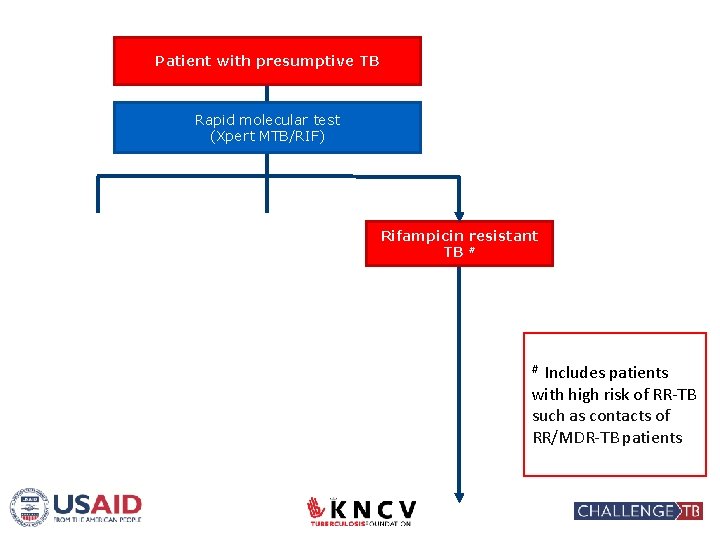
Patient with presumptive TB Rapid molecular test (Xpert MTB/RIF) No TB Rif susceptible TB Appropriate referral/ treatment Standard TB treatment with First Line Drugs Rifampicin resistant TB # Includes patients with high risk of RR-TB such as contacts of RR/MDR-TB patients #

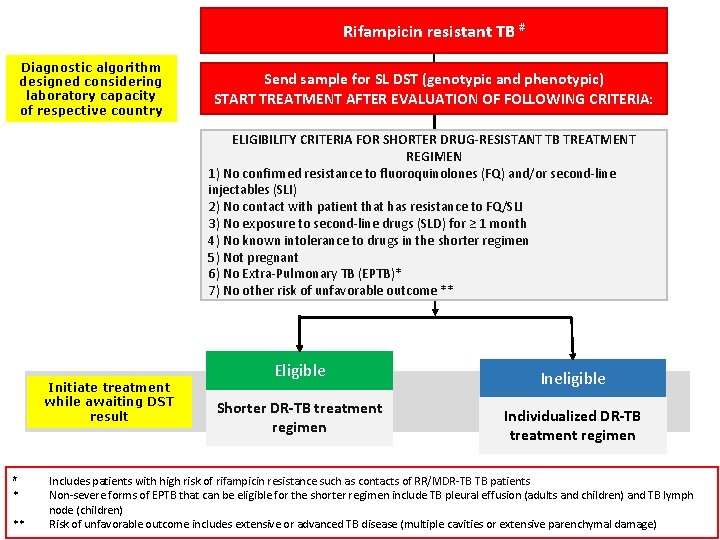
Rifampicin resistant TB # Diagnostic algorithm designed considering laboratory capacity of respective country Send sample for SL DST (genotypic and phenotypic) START TREATMENT AFTER EVALUATION OF FOLLOWING CRITERIA: ELIGIBILITY CRITERIA FOR SHORTER DRUG-RESISTANT TB TREATMENT REGIMEN 1) No confirmed resistance to fluoroquinolones (FQ) and/or second-line injectables (SLI) 2) No contact with patient that has resistance to FQ/SLI 3) No exposure to second-line drugs (SLD) for ≥ 1 month 4) No known intolerance to drugs in the shorter regimen 5) Not pregnant 6) No Extra-Pulmonary TB (EPTB)* 7) No other risk of unfavorable outcome ** Initiate treatment while awaiting DST Initial treatment regimen result # * ** Eligible Shorter DR-TB treatment regimen Ineligible Individualized DR-TB treatment regimen Includes patients with high risk of rifampicin resistance such as contacts of RR/MDR-TB TB patients Non-severe forms of EPTB that can be eligible for the shorter regimen include TB pleural effusion (adults and children) and TB lymph node (children) Risk of unfavorable outcome includes extensive or advanced TB disease (multiple cavities or extensive parenchymal damage)

Initiate treatment Initial treatment regimen while awaiting DST result Regimen adjustment based on • Second-line drug DST results susceptibility testing results & • Treatment tolerance No resistance / intolerance Eligible Ineligible Shorter DR-TB treatment regimen Individualized DR-TB treatment regimen Resistance / intolerance to SLI and/or FQ to SLI and/or FQ Continue shorter DR-TB treatment regimen Change to individualized DR-TB treatment regimen Continue individualized DR-TB treatment regimen No resistance / intolerance to SLI and/or FQ Continue individualized DR-TB treatment whilst consulting experts

Individualized treatment regimens for RR-TB patients ineligible for STR • Challenge TB supports inclusion of new drugs in the core DR-TB regimen for patients not eligible for the STR: • Bdq and Dlm have strong bactericidal and sterilizing activity • Efficacy confirmed in RCTs and is comparable to FLD • Tolerability of both new drugs is well documented in RCTs WHO can not yet fully support the inclusion of new drugs as part of the core MDR-TB regimen while phase III trials are still ongoing

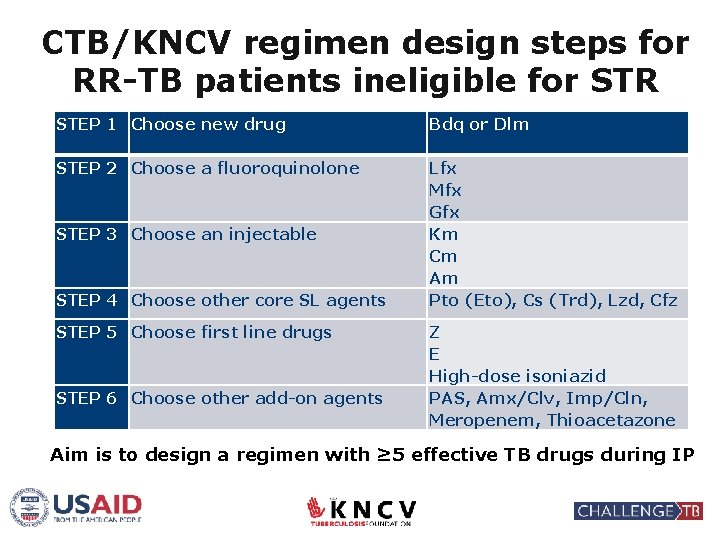
CTB/KNCV regimen design steps for RR-TB patients ineligible for STR STEP 1 Choose new drug STEP 2 Choose a fluoroquinolone STEP 3 Choose an injectable STEP 4 Choose other core SL agents STEP 5 Choose first line drugs STEP 6 Choose other add-on agents Bdq or Dlm Lfx Mfx Gfx Km Cm Am Pto (Eto), Cs (Trd), Lzd, Cfz Z E High-dose isoniazid PAS, Amx/Clv, Imp/Cln, Meropenem, Thioacetazone Aim is to design a regimen with ≥ 5 effective TB drugs during IP

CTB/KNCV documents and tools to support planning and implementation of ND&Rs n n Implementation planning tool Generic programmatic and clinical guide Generic SOPs (under development) Generic training materials (under development)

Right diagnosis, right treatment: Benefits n n n Patients – earlier, effective treatment with lowest possible level of side effects. Reduced costs Health systems - shorter diagnostic delay, and less toxic, shorter and cheaper treatment, leading to better success rates Public health - reduced transmission and reduction in creation of additional DR

Thank you! Acknowledgments: Gunta Dravniece Fraser Wares Agnes Gebhard

 Right product right place right time right price
Right product right place right time right price Right time right place right quantity right quality
Right time right place right quantity right quality Actual diagnosis
Actual diagnosis Medical diagnosis and nursing diagnosis difference
Medical diagnosis and nursing diagnosis difference Nursing diagnosis three parts
Nursing diagnosis three parts Medical diagnosis and nursing diagnosis difference
Medical diagnosis and nursing diagnosis difference Perbedaan diagnosis gizi dan diagnosis medis
Perbedaan diagnosis gizi dan diagnosis medis Charting identification chapter 28
Charting identification chapter 28 Classification of lateral throat form
Classification of lateral throat form Endodontic diagnosis and treatment planning
Endodontic diagnosis and treatment planning Chapter 28 oral diagnosis and treatment planning
Chapter 28 oral diagnosis and treatment planning Patient 2 patient
Patient 2 patient The right man on the right place at the right time
The right man on the right place at the right time Hình ảnh bộ gõ cơ thể búng tay
Hình ảnh bộ gõ cơ thể búng tay Slidetodoc
Slidetodoc