The MONET trial darunavirritonavir monotherapy shows noninferior efficacy



- Slides: 16

The MONET trial: darunavir/ritonavir monotherapy shows non-inferior efficacy to standard HAART, for patients with HIV RNA <50 copies/m. L at baseline JR Arribas, A Horban, J Gerstoft, G Fä F tkenheuer, M Nelson, N Clumeck, F Pulido, A Hill, Y van Delft, C Moecklinghoff, T Stark for the MONET Study Group Oral Late-Breaker presentation at 5 th IAS Conference Cape Town, South Africa, July 2009 #TUAB 106 -LB

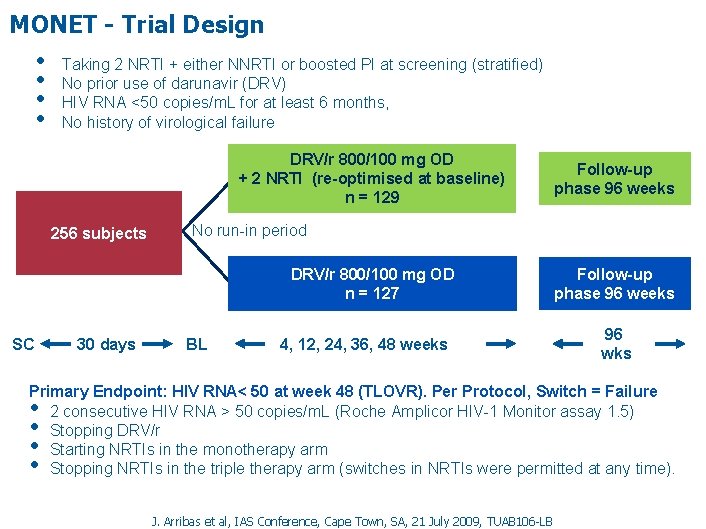
MONET - Trial Design • • Taking 2 NRTI + either NNRTI or boosted PI at screening (stratified) No prior use of darunavir (DRV) HIV RNA <50 copies/m. L for at least 6 months, No history of virological failure DRV/r 800/100 mg OD + 2 NRTI (re-optimised at baseline) n = 129 256 subjects No run-in period DRV/r 800/100 mg OD n = 127 SC 30 days Follow-up phase 96 weeks BL 4, 12, 24, 36, 48 weeks Follow-up phase 96 weeks 96 wks Primary Endpoint: HIV RNA< 50 at week 48 (TLOVR). Per Protocol, Switch = Failure 2 consecutive HIV RNA > 50 copies/m. L (Roche Amplicor HIV-1 Monitor assay 1. 5) Stopping DRV/r Starting NRTIs in the monotherapy arm Stopping NRTIs in the triple therapy arm (switches in NRTIs were permitted at any time). • • J. Arribas et al, IAS Conference, Cape Town, SA, 21 July 2009, TUAB 106 -LB

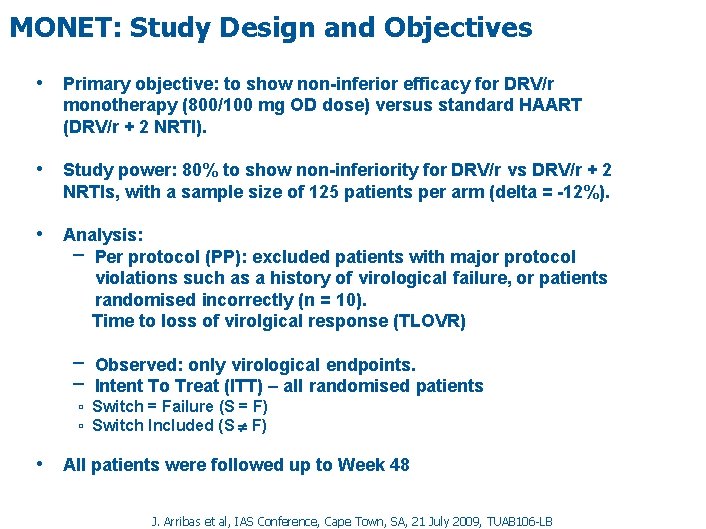
MONET: Study Design and Objectives • Primary objective: to show non-inferior efficacy for DRV/r monotherapy (800/100 mg OD dose) versus standard HAART (DRV/r + 2 NRTI). • Study power: 80% to show non-inferiority for DRV/r vs DRV/r + 2 NRTIs, with a sample size of 125 patients per arm (delta = -12%). • Analysis: − Per protocol (PP): excluded patients with major protocol violations such as a history of virological failure, or patients randomised incorrectly (n = 10). Time to loss of virolgical response (TLOVR) − Observed: only virological endpoints. − Intent To Treat (ITT) – all randomised patients ▫ Switch = Failure (S = F) ▫ Switch Included (S F) • All patients were followed up to Week 48 J. Arribas et al, IAS Conference, Cape Town, SA, 21 July 2009, TUAB 106 -LB

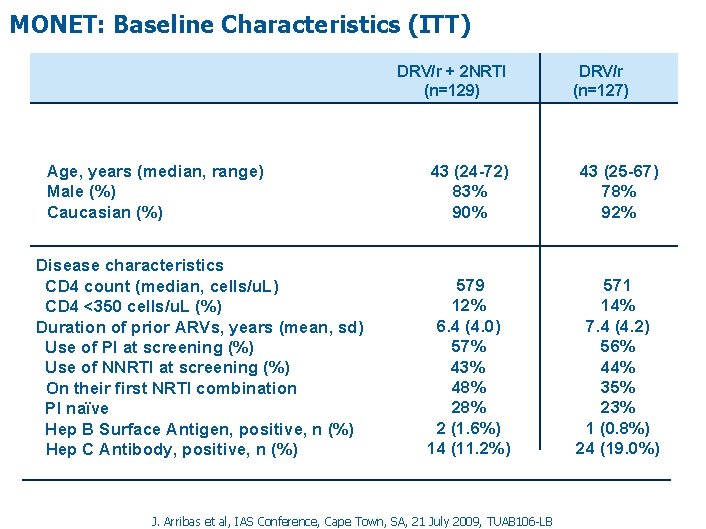
MONET: Baseline Characteristics (ITT) DRV/r + 2 NRTI (n=129) Age, years (median, range) Male (%) Caucasian (%) Disease characteristics CD 4 count (median, cells/u. L) CD 4 <350 cells/u. L (%) Duration of prior ARVs, years (mean, sd) Use of PI at screening (%) Use of NNRTI at screening (%) On their first NRTI combination PI naïve Hep B Surface Antigen, positive, n (%) Hep C Antibody, positive, n (%) DRV/r (n=127) 43 (24 -72) 83% 90% 43 (25 -67) 78% 92% 579 12% 6. 4 (4. 0) 57% 43% 48% 2 (1. 6%) 14 (11. 2%) 571 14% 7. 4 (4. 2) 56% 44% 35% 23% 1 (0. 8%) 24 (19. 0%) J. Arribas et al, IAS Conference, Cape Town, SA, 21 July 2009, TUAB 106 -LB

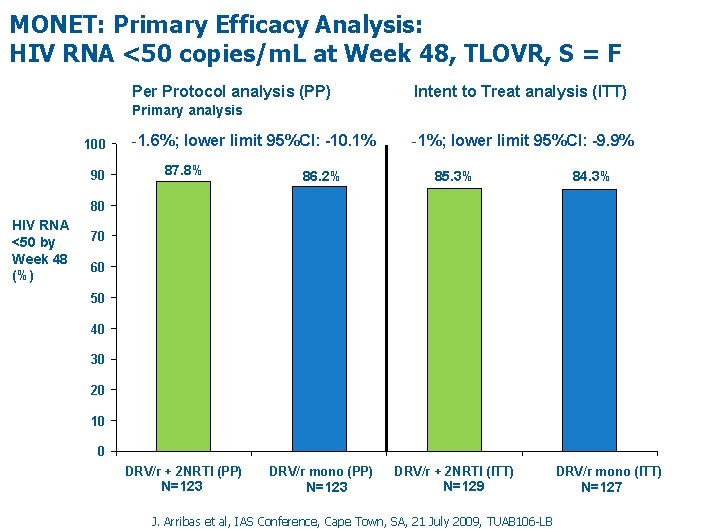
MONET: Primary Efficacy Analysis: HIV RNA <50 copies/m. L at Week 48, TLOVR, S = F Per Protocol analysis (PP) Intent to Treat analysis (ITT) Primary analysis 100 90 -1. 6%; lower limit 95%CI: -10. 1% -1%; lower limit 95%CI: -9. 9% 87. 8% 86. 2% 85. 3% DRV/r + 2 NRTI (PP) DRV/r mono (PP) DRV/r + 2 NRTI (ITT) 84. 3% 80 HIV RNA <50 by Week 48 (%) 70 60 50 40 30 20 10 0 Table EFF 4 -5 N=123 N=129 J. Arribas et al, IAS Conference, Cape Town, SA, 21 July 2009, TUAB 106 -LB DRV/r mono (ITT) N=127

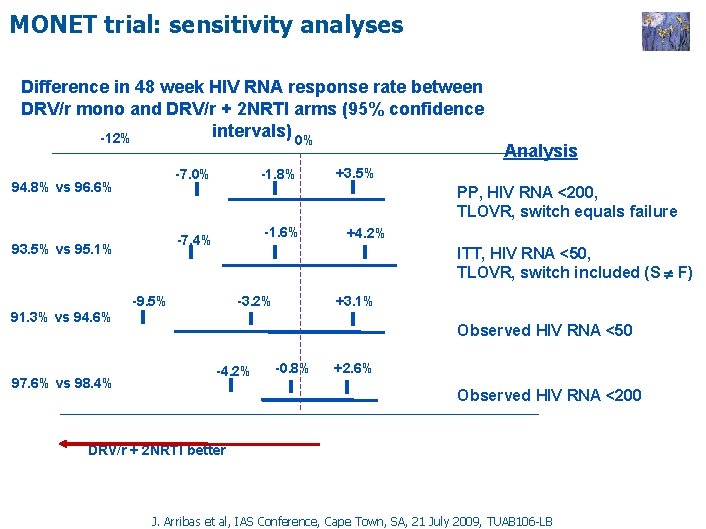
MONET trial: sensitivity analyses Difference in 48 week HIV RNA response rate between DRV/r mono and DRV/r + 2 NRTI arms (95% confidence intervals) 0% -12% -1. 8% -7. 0% 94. 8% vs 96. 6% +3. 5% PP, HIV RNA <200, TLOVR, switch equals failure -1. 6% -7. 4% 93. 5% vs 95. 1% +4. 2% ITT, HIV RNA <50, TLOVR, switch included (S F) -9. 5% -3. 2% +3. 1% 91. 3% vs 94. 6% 97. 6% vs 98. 4% Analysis Observed HIV RNA <50 -4. 2% -0. 8% +2. 6% Observed HIV RNA <200 DRV/r + 2 NRTI better J. Arribas et al, IAS Conference, Cape Town, SA, 21 July 2009, TUAB 106 -LB

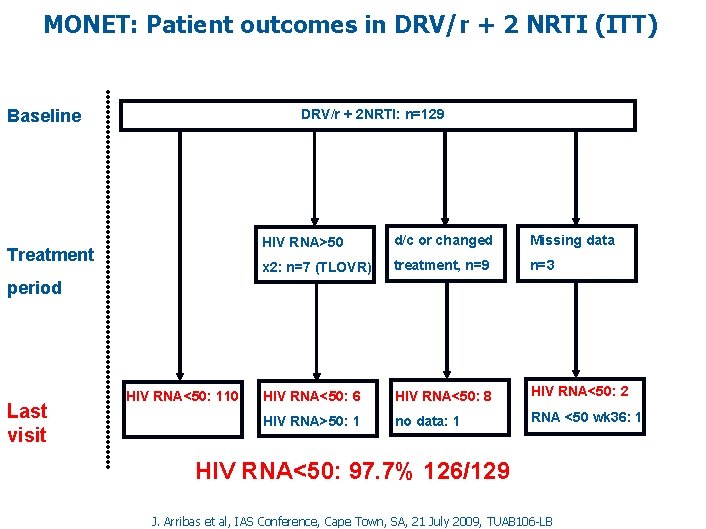
MONET: Patient outcomes in DRV/r + 2 NRTI (ITT) Baseline DRV/r + 2 NRTI: n=129 Treatment HIV RNA>50 d/c or changed Missing data x 2: n=7 (TLOVR) treatment, n=9 n=3 HIV RNA<50: 6 HIV RNA<50: 8 HIV RNA<50: 2 HIV RNA>50: 1 no data: 1 RNA <50 wk 36: 1 period Last visit HIV RNA<50: 110 HIV RNA<50: 97. 7% 126/129 J. Arribas et al, IAS Conference, Cape Town, SA, 21 July 2009, TUAB 106 -LB

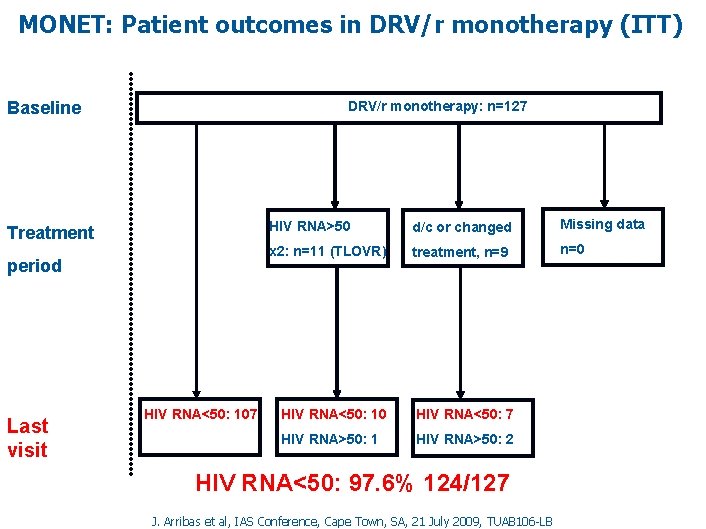
MONET: Patient outcomes in DRV/r monotherapy (ITT) DRV/r monotherapy: n=127 Baseline Treatment period Last visit HIV RNA<50: 107 HIV RNA>50 d/c or changed Missing data x 2: n=11 (TLOVR) treatment, n=9 n=0 HIV RNA<50: 10 HIV RNA<50: 7 HIV RNA>50: 1 HIV RNA>50: 2 HIV RNA<50: 97. 6% 124/127 J. Arribas et al, IAS Conference, Cape Town, SA, 21 July 2009, TUAB 106 -LB

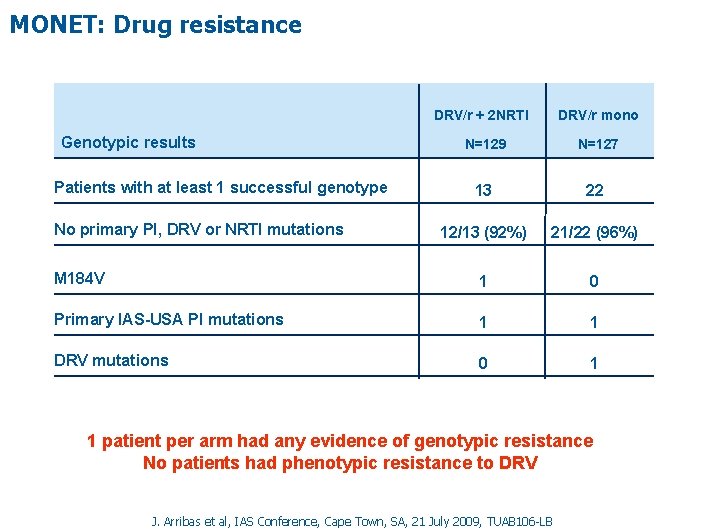
MONET: Drug resistance DRV/r + 2 NRTI Genotypic results DRV/r mono N=129 N=127 13 22 12/13 (92%) 21/22 (96%) M 184 V 1 0 Primary IAS-USA PI mutations 1 1 DRV mutations 0 1 Patients with at least 1 successful genotype No primary PI, DRV or NRTI mutations 1 patient per arm had any evidence of genotypic resistance No patients had phenotypic resistance to DRV J. Arribas et al, IAS Conference, Cape Town, SA, 21 July 2009, TUAB 106 -LB

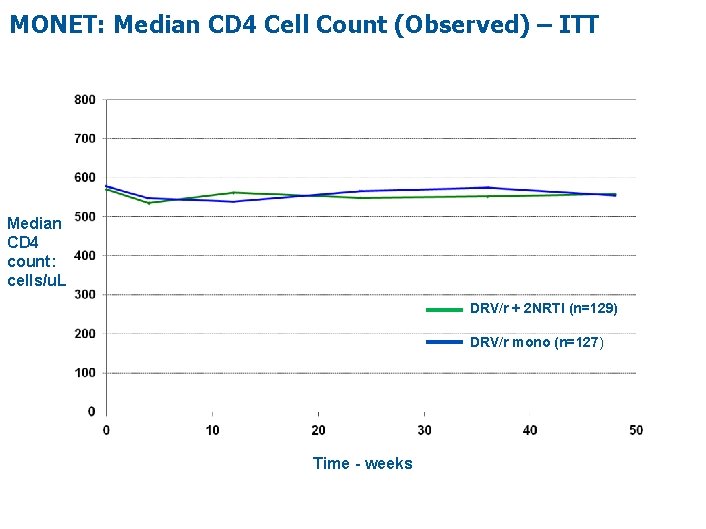
MONET: Median CD 4 Cell Count (Observed) – ITT Median CD 4 count: cells/u. L DRV/r + 2 NRTI (n=129) DRV/r mono (n=127) Time - weeks

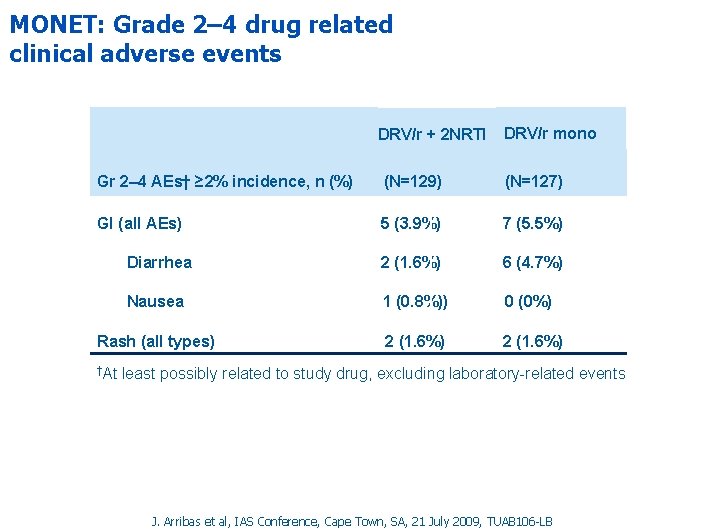
MONET: Grade 2– 4 drug related clinical adverse events DRV/r + 2 NRTI DRV/r mono Gr 2– 4 AEs† ≥ 2% incidence, n (%) (N=129) (N=127) GI (all AEs) 5 (3. 9%) 7 (5. 5%) Diarrhea 2 (1. 6%) 6 (4. 7%) Nausea 1 (0. 8%)) 0 (0%) Rash (all types) 2 (1. 6%) †At least possibly related to study drug, excluding laboratory-related events J. Arribas et al, IAS Conference, Cape Town, SA, 21 July 2009, TUAB 106 -LB

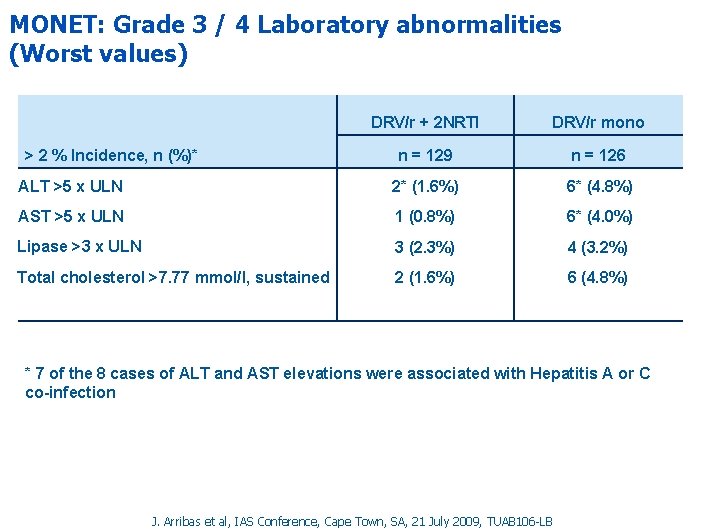
MONET: Grade 3 / 4 Laboratory abnormalities (Worst values) DRV/r + 2 NRTI DRV/r mono n = 129 n = 126 ALT >5 x ULN 2* (1. 6%) 6* (4. 8%) AST >5 x ULN 1 (0. 8%) 6* (4. 0%) Lipase >3 x ULN 3 (2. 3%) 4 (3. 2%) Total cholesterol >7. 77 mmol/l, sustained 2 (1. 6%) 6 (4. 8%) > 2 % Incidence, n (%)* * 7 of the 8 cases of ALT and AST elevations were associated with Hepatitis A or C co-infection J. Arribas et al, IAS Conference, Cape Town, SA, 21 July 2009, TUAB 106 -LB

Conclusions § Darunavir/ritonavir monotherapy showed consistently non-inferior efficacy versus triple antiretroviral drug treatment at Week 48. § Most elevations in HIV RNA were low level (50 -400 copies/m. L), and patients were re-suppressed <50 copies/m. L at last visit, either on the original randomised treatment or with intensified treatment. § There were no patients with phenotypic resistance to darunavir during the trial – one patient per arm showed at least one genotypic PI mutation. § No new or unexpected safety signals were detected (details submitted to EACS and AELD conferences). J. Arribas et al, IAS Conference, Cape Town, SA, 21 July 2009, TUAB 106 -LB

Efficacy of darunavir/ritonavir as single-drug maintenance therapy in patients with HIV-1 viral suppression: a randomized open-label non-inferiority trial, MONOI-ANRS 136 C. Katlama et al #WELBB 102 WEDNESDAY. LATE BREAKERS TRACK B. Session Room 1. 13: 10 J. Arribas et al, IAS Conference, Cape Town, SA, 21 July 2009, TUAB 106 -LB

MONET: Acknowledgements Thanks to all the 256 patients who participated in the MONET trial, plus the investigators and study monitors J. Arribas et al, IAS Conference, Cape Town, SA, 21 July 2009, TUAB 106 -LB

MONET: Acknowledgements Participating centers: Austria: A. Rieger, N. Vetter Belgium: N. Clumeck, E. Florence Switzerland: P. Vernazza Germany: G. Fätkenheuer, A. Stoehr, W. Schmidt, M, Stoll, C. Stephan Denmark: J. Gerstoft, C. Pedersen, L. Mathiesen Spain: B. Clotet, F. Pulido, J. Arribas, J. Gatell, J. Iribarren, R. Rubio, J. Pasquau United Kingdom: M. Johnson, B. Peters, M. Nelson, A. Winston, Hungary: D. Banhegyi Israel: S. Maayan Italy: A. Lazzarin, A. Antinori, F. Suter, A. D‘Arminio Monforte, G. Carosi Poland: A. Horban Portugal: F. Antunes, R. Marques Russia: N Zakharova, V. Pokrovsky The authors would like to thank the patients and their families for their participation and support during the study, and the MONET study team and co-investigators for their collaboration. This study was sponsored by Tibotec, a division of Janssen-Cilag. J. Arribas et al, IAS Conference, Cape Town, SA, 21 July 2009, TUAB 106 -LB
 When god shows up he shows off
When god shows up he shows off Vaccine efficacy
Vaccine efficacy Testosterone injection dosage chart
Testosterone injection dosage chart Slidetodoc.com
Slidetodoc.com Collective teacher efficacy
Collective teacher efficacy What is role efficacy
What is role efficacy Vaccine efficacy
Vaccine efficacy Optimal confidence
Optimal confidence Efficacy potency
Efficacy potency Collective teacher efficacy
Collective teacher efficacy Efficacy definition pharmacology
Efficacy definition pharmacology Luminous efficacy comparison chart
Luminous efficacy comparison chart Drug efficacy
Drug efficacy Self-efficacy theory
Self-efficacy theory Albert bandura self-efficacy
Albert bandura self-efficacy Potency vs efficacy
Potency vs efficacy Drug efficacy
Drug efficacy