Switch to DRVr monotherapy MONOI MONET PROTEA MONET

- Slides: 10

Switch to DRV/r monotherapy § MONOI § MONET § PROTEA

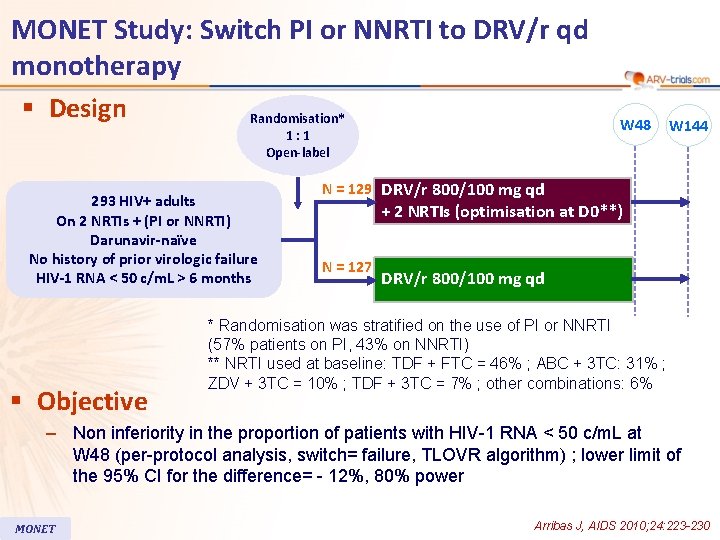
MONET Study: Switch PI or NNRTI to DRV/r qd monotherapy § Design Randomisation* 1: 1 Open-label 293 HIV+ adults On 2 NRTIs + (PI or NNRTI) Darunavir-naïve No history of prior virologic failure HIV-1 RNA < 50 c/m. L > 6 months § Objective W 48 W 144 N = 129 DRV/r 800/100 mg qd + 2 NRTIs (optimisation at D 0**) N = 127 DRV/r 800/100 mg qd * Randomisation was stratified on the use of PI or NNRTI (57% patients on PI, 43% on NNRTI) ** NRTI used at baseline: TDF + FTC = 46% ; ABC + 3 TC: 31% ; ZDV + 3 TC = 10% ; TDF + 3 TC = 7% ; other combinations: 6% – Non inferiority in the proportion of patients with HIV-1 RNA < 50 c/m. L at W 48 (per-protocol analysis, switch= failure, TLOVR algorithm) ; lower limit of the 95% CI for the difference= - 12%, 80% power MONET Arribas J, AIDS 2010; 24: 223 -230

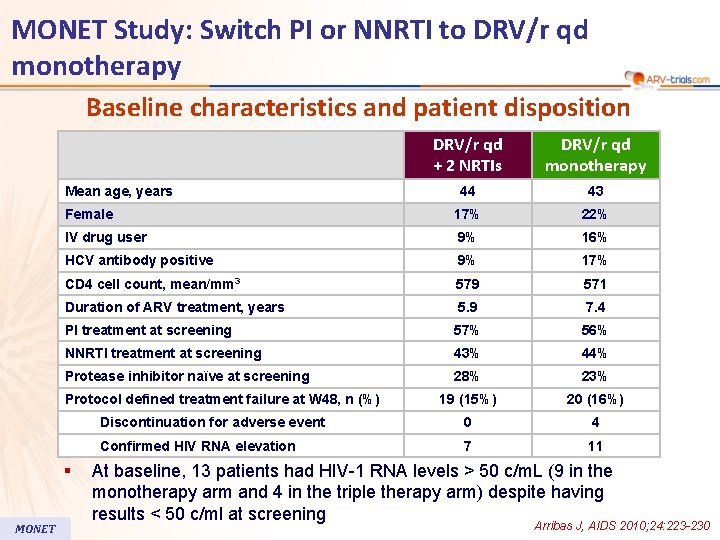
MONET Study: Switch PI or NNRTI to DRV/r qd monotherapy Baseline characteristics and patient disposition DRV/r qd + 2 NRTIs DRV/r qd monotherapy 44 43 Female 17% 22% IV drug user 9% 16% HCV antibody positive 9% 17% CD 4 cell count, mean/mm 3 579 571 Duration of ARV treatment, years 5. 9 7. 4 PI treatment at screening 57% 56% NNRTI treatment at screening 43% 44% Protease inhibitor naïve at screening 28% 23% 19 (15%) 20 (16%) Discontinuation for adverse event 0 4 Confirmed HIV RNA elevation 7 11 Mean age, years Protocol defined treatment failure at W 48, n (%) § MONET At baseline, 13 patients had HIV-1 RNA levels > 50 c/m. L (9 in the monotherapy arm and 4 in the triple therapy arm) despite having results < 50 c/ml at screening Arribas J, AIDS 2010; 24: 223 -230

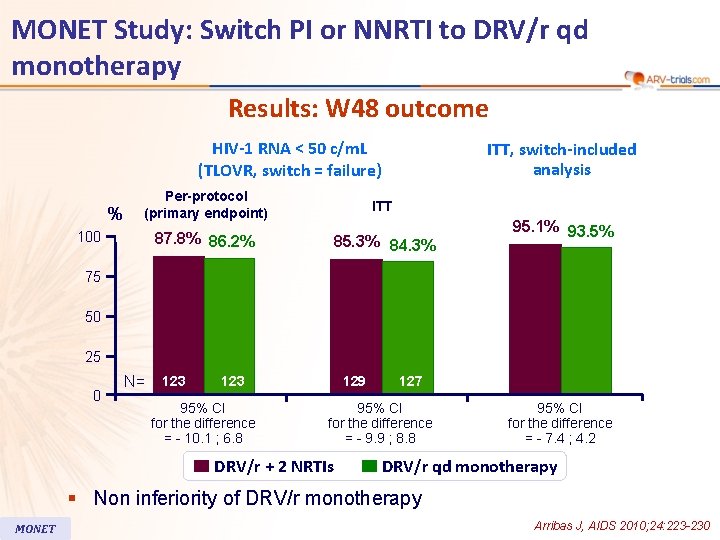
MONET Study: Switch PI or NNRTI to DRV/r qd monotherapy Results: W 48 outcome HIV-1 RNA < 50 c/m. L (TLOVR, switch = failure) % ITT, switch-included analysis Per-protocol (primary endpoint) ITT 87. 8% 86. 2% 85. 3% 84. 3% 100 95. 1% 93. 5% 75 50 25 0 N= 123 95% CI for the difference = - 10. 1 ; 6. 8 129 127 95% CI for the difference = - 9. 9 ; 8. 8 DRV/r + 2 NRTIs 95% CI for the difference = - 7. 4 ; 4. 2 DRV/r qd monotherapy § Non inferiority of DRV/r monotherapy MONET Arribas J, AIDS 2010; 24: 223 -230

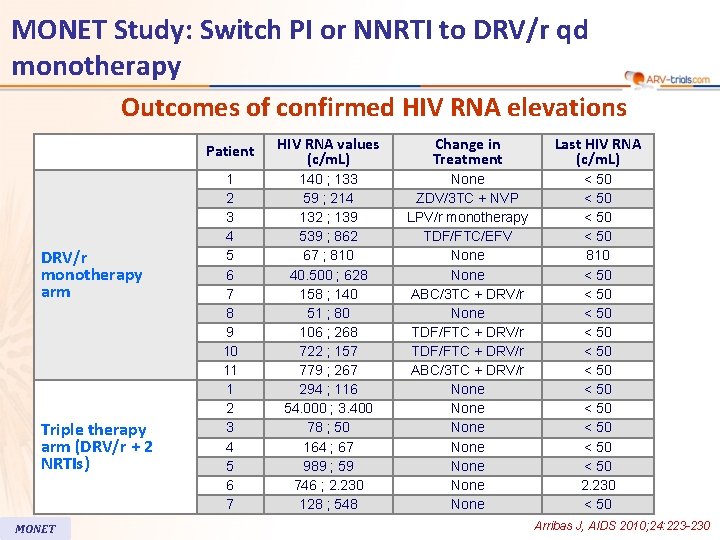
MONET Study: Switch PI or NNRTI to DRV/r qd monotherapy Outcomes of confirmed HIV RNA elevations DRV/r monotherapy arm Triple therapy arm (DRV/r + 2 NRTIs) MONET Patient HIV RNA values (c/m. L) Change in Treatment Last HIV RNA (c/m. L) 1 2 3 4 5 6 7 8 9 10 11 1 2 3 4 5 6 7 140 ; 133 59 ; 214 132 ; 139 539 ; 862 67 ; 810 40. 500 ; 628 158 ; 140 51 ; 80 106 ; 268 722 ; 157 779 ; 267 294 ; 116 54. 000 ; 3. 400 78 ; 50 164 ; 67 989 ; 59 746 ; 2. 230 128 ; 548 None ZDV/3 TC + NVP LPV/r monotherapy TDF/FTC/EFV None ABC/3 TC + DRV/r None TDF/FTC + DRV/r ABC/3 TC + DRV/r None None < 50 810 < 50 < 50 < 50 2. 230 < 50 Arribas J, AIDS 2010; 24: 223 -230

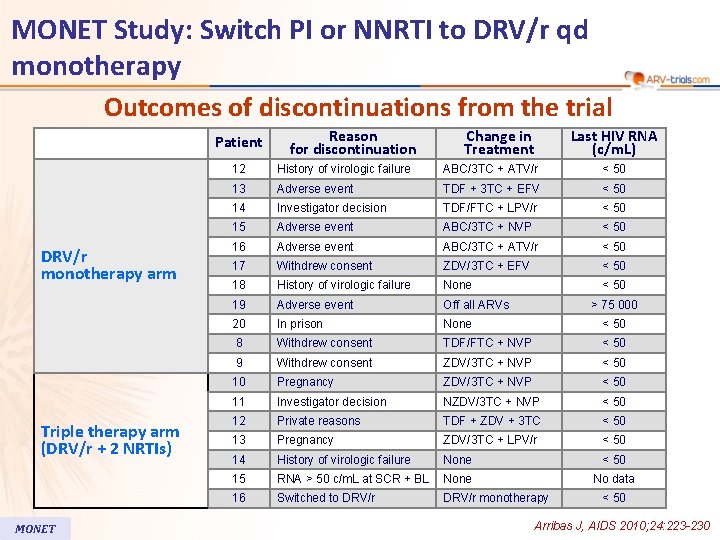
MONET Study: Switch PI or NNRTI to DRV/r qd monotherapy Outcomes of discontinuations from the trial Patient DRV/r monotherapy arm Triple therapy arm (DRV/r + 2 NRTIs) MONET Reason for discontinuation Change in Treatment Last HIV RNA (c/m. L) 12 History of virologic failure ABC/3 TC + ATV/r < 50 13 Adverse event TDF + 3 TC + EFV < 50 14 Investigator decision TDF/FTC + LPV/r < 50 15 Adverse event ABC/3 TC + NVP < 50 16 Adverse event ABC/3 TC + ATV/r < 50 17 Withdrew consent ZDV/3 TC + EFV < 50 18 History of virologic failure None < 50 19 Adverse event Off all ARVs 20 In prison None < 50 8 Withdrew consent TDF/FTC + NVP < 50 9 Withdrew consent ZDV/3 TC + NVP < 50 10 Pregnancy ZDV/3 TC + NVP < 50 11 Investigator decision NZDV/3 TC + NVP < 50 12 Private reasons TDF + ZDV + 3 TC < 50 13 Pregnancy ZDV/3 TC + LPV/r < 50 14 History of virologic failure None < 50 15 RNA > 50 c/m. L at SCR + BL None No data 16 Switched to DRV/r monotherapy > 75 000 < 50 Arribas J, AIDS 2010; 24: 223 -230

MONET Study: Switch PI or NNRTI to DRV/r qd monotherapy Other endpoints § In multivariate analysis, hepatitis C co-infection was a significant predictor of confirmed HIV RNA elevations (p < 0. 01) § Resistance data: Genotype was available for 35/61 patients with HIV RNA > 50 c/m. L (22 in the monotherapy group and 13 in the triple therapy group) – Resistance mutations to PI in 1 one patient in each arm, with no phenotypic resistance to DRV. HIV-1 RNA returned to < 50 c/m. L without changing therapy in both patients § Most common grade 2 to 4 adverse events (AE) were gastrointestinal § Serious AE were seen in 9 patients in each group § Discontinuation for AE by W 48 occurred in 8 patients in the monotherapy group and 3 in the triple therapy group § Grade 1 to 4 nervous system AE were seen in 16% of patients in each group, and Grade 1 to 4 psychiatric AE in 9% of patients in each group § There were more haematological abnormalities in the triple therapy arm, related to zidovudine MONET Arribas J, AIDS 2010; 24: 223 -230

MONET Study: Switch PI or NNRTI to DRV/r qd monotherapy § Conclusions from W 48 data – In patients with virologic suppression on standard triple therapy (2 NRTIs + 1 NNRTI or 1 PI), once-daily DRV/r monotherapy has shown non inferior HIV RNA suppression at week 48 compared with a standard therapy of 2 NRTIs + once-daily DRV/r – A switch to once-daily DRV/r monotherapy can be considered in patients who have HIV RNA < 50 c/m. L for more than 6 months on other treatments and no history of virologic failure, but wish to avoid toxicities related to other ARVs MONET Arribas J, AIDS 2010; 24: 223 -230

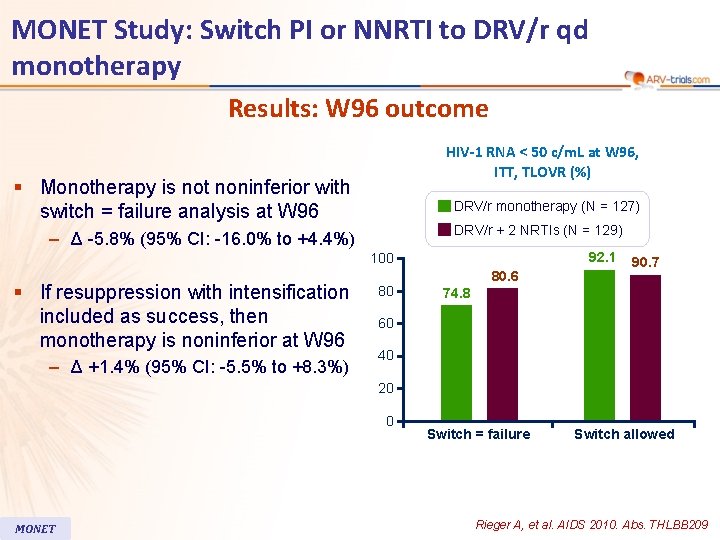
MONET Study: Switch PI or NNRTI to DRV/r qd monotherapy Results: W 96 outcome HIV-1 RNA < 50 c/m. L at W 96, ITT, TLOVR (%) § Monotherapy is not noninferior with switch = failure analysis at W 96 DRV/r monotherapy (N = 127) DRV/r + 2 NRTIs (N = 129) – Δ -5. 8% (95% CI: -16. 0% to +4. 4%) 92. 1 100 § If resuppression with intensification included as success, then monotherapy is noninferior at W 96 – Δ +1. 4% (95% CI: -5. 5% to +8. 3%) 80 80. 6 90. 7 74. 8 60 40 20 0 MONET Switch = failure Switch allowed Rieger A, et al. AIDS 2010. Abs. THLBB 209

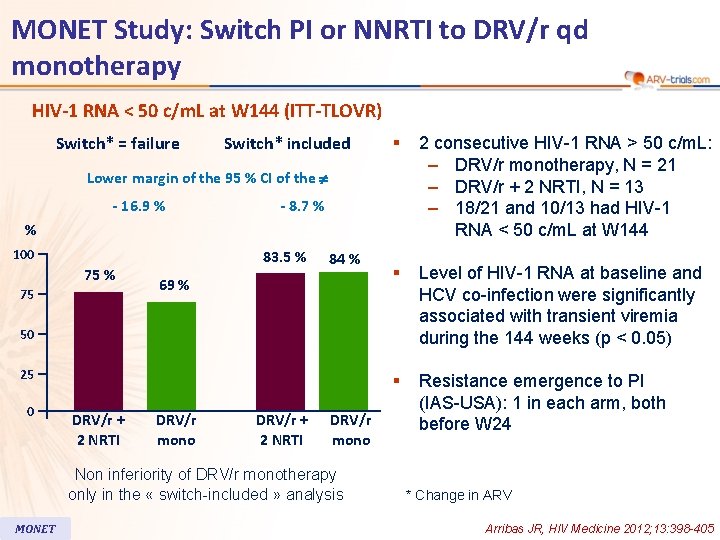
MONET Study: Switch PI or NNRTI to DRV/r qd monotherapy HIV-1 RNA < 50 c/m. L at W 144 (ITT-TLOVR) Switch* = failure Switch* included § 2 consecutive HIV-1 RNA > 50 c/m. L: – DRV/r monotherapy, N = 21 – DRV/r + 2 NRTI, N = 13 – 18/21 and 10/13 had HIV-1 RNA < 50 c/m. L at W 144 § Level of HIV-1 RNA at baseline and HCV co-infection were significantly associated with transient viremia during the 144 weeks (p < 0. 05) § Resistance emergence to PI (IAS-USA): 1 in each arm, both before W 24 Lower margin of the 95 % CI of the - 16. 9 % - 8. 7 % % 100 75 % 75 83. 5 % 84 % 69 % 50 25 0 DRV/r + 2 NRTI DRV/r mono Non inferiority of DRV/r monotherapy only in the « switch-included » analysis MONET * Change in ARV Arribas JR, HIV Medicine 2012; 13: 398 -405