Switch to DRVr monotherapy MONOI MONET PROTEA MONOI

- Slides: 9

Switch to DRV/r monotherapy § MONOI § MONET § PROTEA

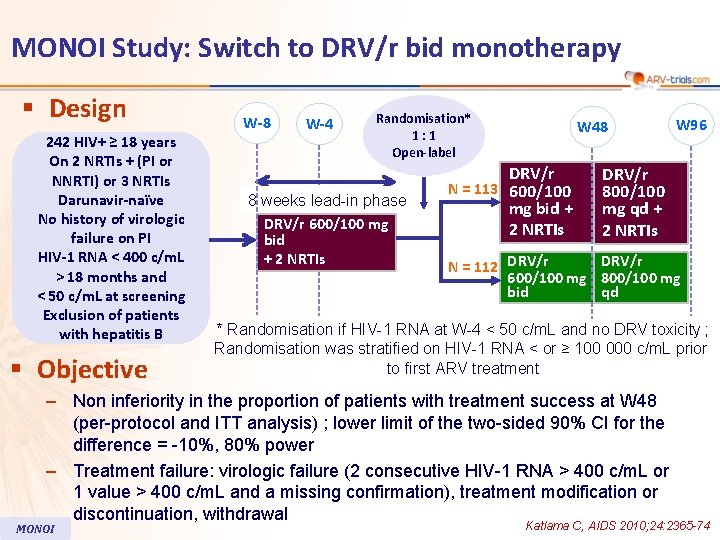
MONOI Study: Switch to DRV/r bid monotherapy § Design 242 HIV+ ≥ 18 years On 2 NRTIs + (PI or NNRTI) or 3 NRTIs Darunavir-naïve No history of virologic failure on PI HIV-1 RNA < 400 c/m. L > 18 months and < 50 c/m. L at screening Exclusion of patients with hepatitis B § Objective W-8 W-4 Randomisation* 1: 1 Open-label 8 weeks lead-in phase DRV/r 600/100 mg bid + 2 NRTIs W 48 DRV/r N = 113 600/100 mg bid + 2 NRTIs DRV/r 800/100 mg qd + 2 NRTIs DRV/r N = 112 DRV/r 600/100 mg 800/100 mg bid qd * Randomisation if HIV-1 RNA at W-4 < 50 c/m. L and no DRV toxicity ; Randomisation was stratified on HIV-1 RNA < or ≥ 100 000 c/m. L prior to first ARV treatment – Non inferiority in the proportion of patients with treatment success at W 48 (per-protocol and ITT analysis) ; lower limit of the two-sided 90% CI for the difference = -10%, 80% power – Treatment failure: virologic failure (2 consecutive HIV-1 RNA > 400 c/m. L or 1 value > 400 c/m. L and a missing confirmation), treatment modification or discontinuation, withdrawal MONOI W 96 Katlama C, AIDS 2010; 24: 2365 -74

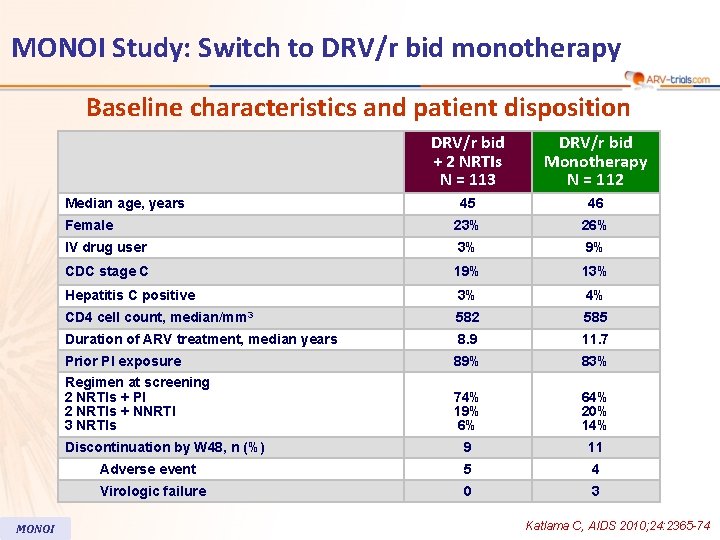
MONOI Study: Switch to DRV/r bid monotherapy Baseline characteristics and patient disposition DRV/r bid + 2 NRTIs N = 113 DRV/r bid Monotherapy N = 112 45 46 Female 23% 26% IV drug user 3% 9% CDC stage C 19% 13% Hepatitis C positive 3% 4% CD 4 cell count, median/mm 3 582 585 Duration of ARV treatment, median years 8. 9 11. 7 Prior PI exposure 89% 83% Regimen at screening 2 NRTIs + PI 2 NRTIs + NNRTI 3 NRTIs 74% 19% 6% 64% 20% 14% 9 11 Adverse event 5 4 Virologic failure 0 3 Median age, years Discontinuation by W 48, n (%) MONOI Katlama C, AIDS 2010; 24: 2365 -74

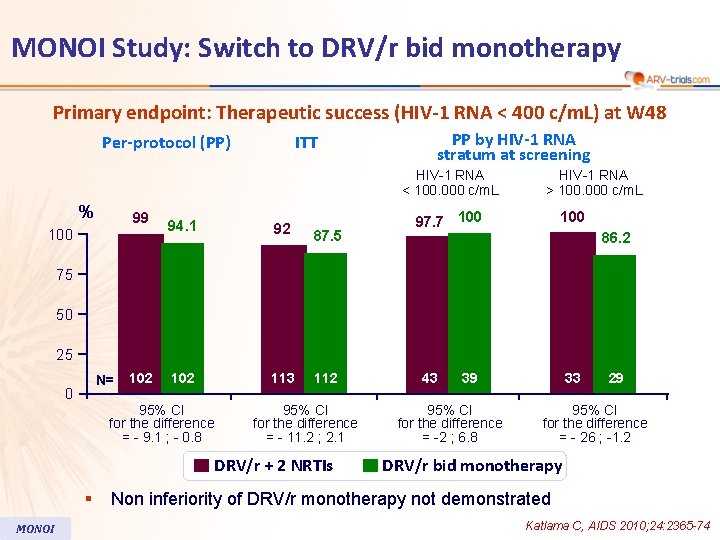
MONOI Study: Switch to DRV/r bid monotherapy Primary endpoint: Therapeutic success (HIV-1 RNA < 400 c/m. L) at W 48 Per-protocol (PP) ITT PP by HIV-1 RNA stratum at screening HIV-1 RNA < 100. 000 c/m. L % 99 100 94. 1 92 87. 5 102 113 112 HIV-1 RNA > 100. 000 c/m. L 97. 7 100 86. 2 75 50 25 N= 0 102 95% CI for the difference = - 9. 1 ; - 0. 8 95% CI for the difference = - 11. 2 ; 2. 1 DRV/r + 2 NRTIs § MONOI 43 39 95% CI for the difference = -2 ; 6. 8 33 29 95% CI for the difference = - 26 ; -1. 2 DRV/r bid monotherapy Non inferiority of DRV/r monotherapy not demonstrated Katlama C, AIDS 2010; 24: 2365 -74

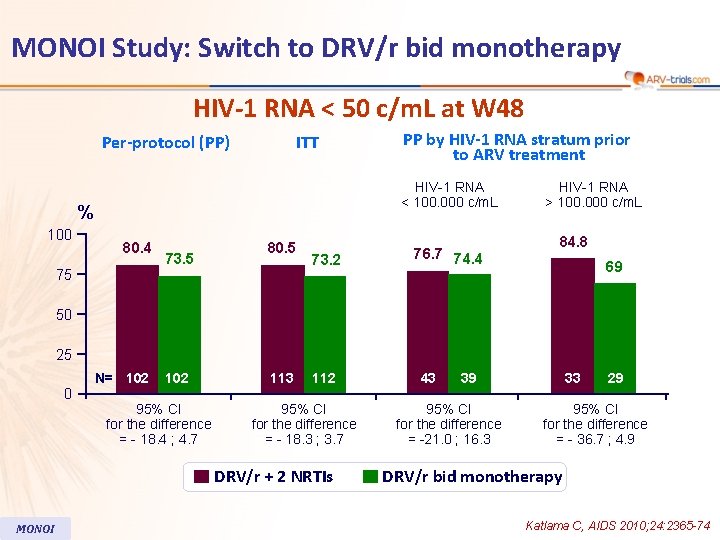
MONOI Study: Switch to DRV/r bid monotherapy HIV-1 RNA < 50 c/m. L at W 48 Per-protocol (PP) ITT HIV-1 RNA < 100. 000 c/m. L % 100 PP by HIV-1 RNA stratum prior to ARV treatment 80. 4 75 73. 5 80. 5 73. 2 76. 7 74. 4 HIV-1 RNA > 100. 000 c/m. L 84. 8 69 50 25 0 N= 102 95% CI for the difference = - 18. 4 ; 4. 7 113 112 95% CI for the difference = - 18. 3 ; 3. 7 DRV/r + 2 NRTIs MONOI 43 39 95% CI for the difference = -21. 0 ; 16. 3 33 29 95% CI for the difference = - 36. 7 ; 4. 9 DRV/r bid monotherapy Katlama C, AIDS 2010; 24: 2365 -74

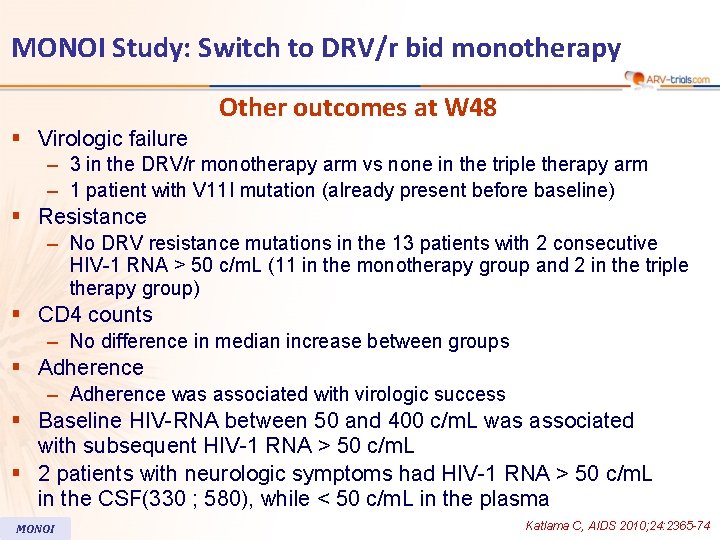
MONOI Study: Switch to DRV/r bid monotherapy Other outcomes at W 48 § Virologic failure – 3 in the DRV/r monotherapy arm vs none in the triple therapy arm – 1 patient with V 11 I mutation (already present before baseline) § Resistance – No DRV resistance mutations in the 13 patients with 2 consecutive HIV-1 RNA > 50 c/m. L (11 in the monotherapy group and 2 in the triple therapy group) § CD 4 counts – No difference in median increase between groups § Adherence – Adherence was associated with virologic success § Baseline HIV-RNA between 50 and 400 c/m. L was associated with subsequent HIV-1 RNA > 50 c/m. L § 2 patients with neurologic symptoms had HIV-1 RNA > 50 c/m. L in the CSF(330 ; 580), while < 50 c/m. L in the plasma MONOI Katlama C, AIDS 2010; 24: 2365 -74

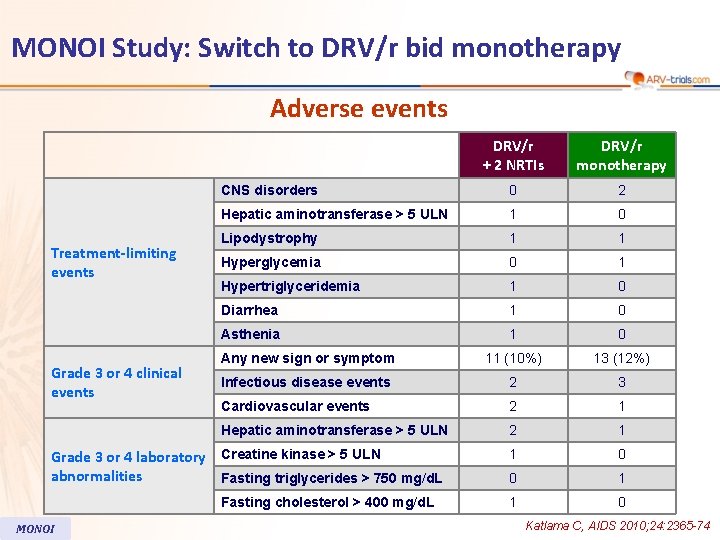
MONOI Study: Switch to DRV/r bid monotherapy Adverse events Treatment-limiting events Grade 3 or 4 clinical events DRV/r + 2 NRTIs DRV/r monotherapy CNS disorders 0 2 Hepatic aminotransferase > 5 ULN 1 0 Lipodystrophy 1 1 Hyperglycemia 0 1 Hypertriglyceridemia 1 0 Diarrhea 1 0 Asthenia 1 0 Any new sign or symptom 11 (10%) 13 (12%) Infectious disease events 2 3 Cardiovascular events 2 1 Hepatic aminotransferase > 5 ULN 2 1 1 0 0 1 1 0 Grade 3 or 4 laboratory Creatine kinase > 5 ULN abnormalities Fasting triglycerides > 750 mg/d. L Fasting cholesterol > 400 mg/d. L MONOI Katlama C, AIDS 2010; 24: 2365 -74

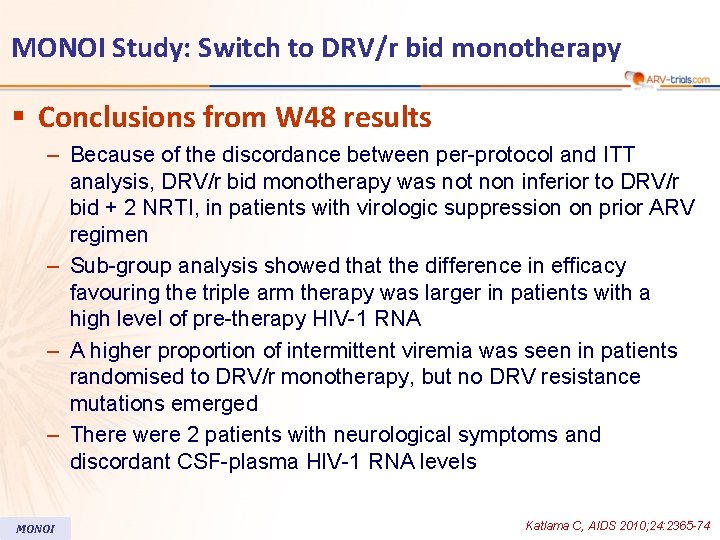
MONOI Study: Switch to DRV/r bid monotherapy § Conclusions from W 48 results – Because of the discordance between per-protocol and ITT analysis, DRV/r bid monotherapy was not non inferior to DRV/r bid + 2 NRTI, in patients with virologic suppression on prior ARV regimen – Sub-group analysis showed that the difference in efficacy favouring the triple arm therapy was larger in patients with a high level of pre-therapy HIV-1 RNA – A higher proportion of intermittent viremia was seen in patients randomised to DRV/r monotherapy, but no DRV resistance mutations emerged – There were 2 patients with neurological symptoms and discordant CSF-plasma HIV-1 RNA levels MONOI Katlama C, AIDS 2010; 24: 2365 -74

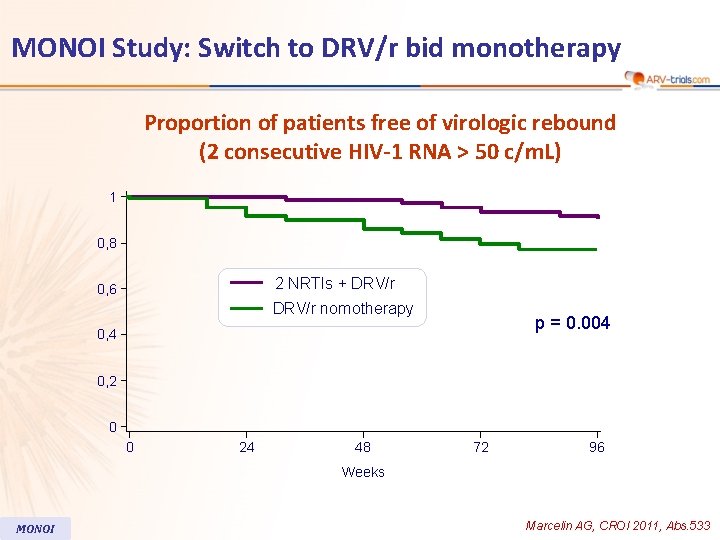
MONOI Study: Switch to DRV/r bid monotherapy Proportion of patients free of virologic rebound (2 consecutive HIV-1 RNA > 50 c/m. L) 1 0, 8 2 NRTIs + DRV/r 0, 6 DRV/r nomotherapy p = 0. 004 0, 2 0 0 24 48 72 96 Weeks MONOI Marcelin AG, CROI 2011, Abs. 533