NonInferior Efficacy of Dolutegravir DTG Plus Lamivudine 3






- Slides: 22

Non-Inferior Efficacy of Dolutegravir (DTG) Plus Lamivudine (3 TC) vs DTG Plus Tenofovir/Emtricitabine (TDF/FTC) Fixed-Dose Combination in Antiretroviral Treatment–Naive Adults With HIV-1 Infection—Week 48 Results From the GEMINI Studies P. Cahn, 1 J. Sierra Madero, 2 J. Arribas, 3 A. Antinori, 4 R. Ortiz, 5 A. Clarke, 6 C. -C. Hung, 7 J. Rockstroh, 8 P. -M. Girard, 9 C. Man, 10 J. Sievers, 11 A. Currie, 12 M. Underwood, 10 A. Tenorio, 10 K. Pappa, 10 B. Wynne, 10 M. Gartland, 10 M. Aboud, 11 K. Smith 10 1 Fundación Huésped, Buenos Aires, Argentina; 2 Instituto Nacional de Ciencias Médicas y Nutrición Salvador Zubirán, Mexico City, Mexico; 3 Hospital La Paz, Madrid, Spain; 4 Istituto Nazionale per le Malattie Infettive Lazzaro Spallanzani, Rome, Italy; 5 Bliss Healthcare Services, Orlando, FL, USA; 6 Royal Sussex County Hospital, Brighton, UK; 7 National Taiwan University Hospital, Taipei, Taiwan, Province of China; 8 Rheinische Friedrich-Wilhelms Universität, Bonn, Germany; 9 Hôpital Saint Antoine, Paris, France; 10 Vii. V Healthcare, Research Triangle Park, NC, USA; 11 Vii. V Healthcare, Brentford, UK; 12 Glaxo. Smith. Kline, Stockley Park, UK 22 nd International AIDS Conference; July 23 -27, 2018; Amsterdam, the Netherlands

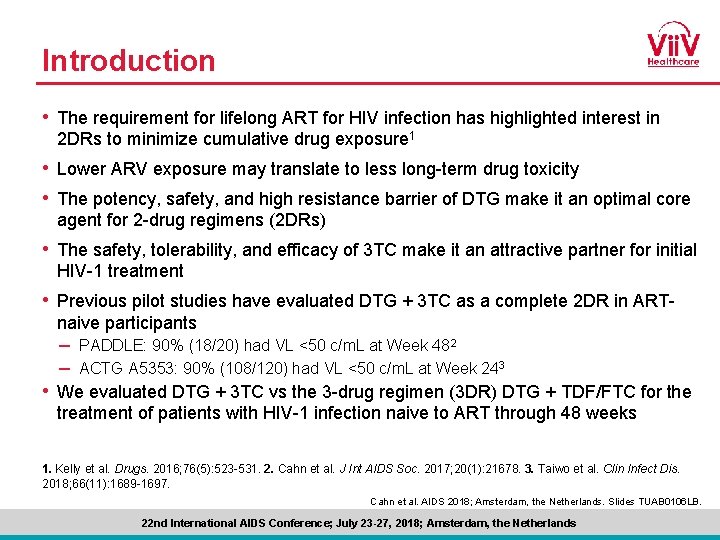
Introduction • The requirement for lifelong ART for HIV infection has highlighted interest in 2 DRs to minimize cumulative drug exposure 1 • Lower ARV exposure may translate to less long-term drug toxicity • The potency, safety, and high resistance barrier of DTG make it an optimal core agent for 2 -drug regimens (2 DRs) • The safety, tolerability, and efficacy of 3 TC make it an attractive partner for initial HIV-1 treatment • Previous pilot studies have evaluated DTG + 3 TC as a complete 2 DR in ARTnaive participants – PADDLE: 90% (18/20) had VL <50 c/m. L at Week 482 – ACTG A 5353: 90% (108/120) had VL <50 c/m. L at Week 243 • We evaluated DTG + 3 TC vs the 3 -drug regimen (3 DR) DTG + TDF/FTC for the treatment of patients with HIV-1 infection naive to ART through 48 weeks 1. Kelly et al. Drugs. 2016; 76(5): 523 -531. 2. Cahn et al. J Int AIDS Soc. 2017; 20(1): 21678. 3. Taiwo et al. Clin Infect Dis. 2018; 66(11): 1689 -1697. Cahn et al. AIDS 2018; Amsterdam, the Netherlands. Slides TUAB 0106 LB. 22 nd International AIDS Conference; July 23 -27, 2018; Amsterdam, the Netherlands

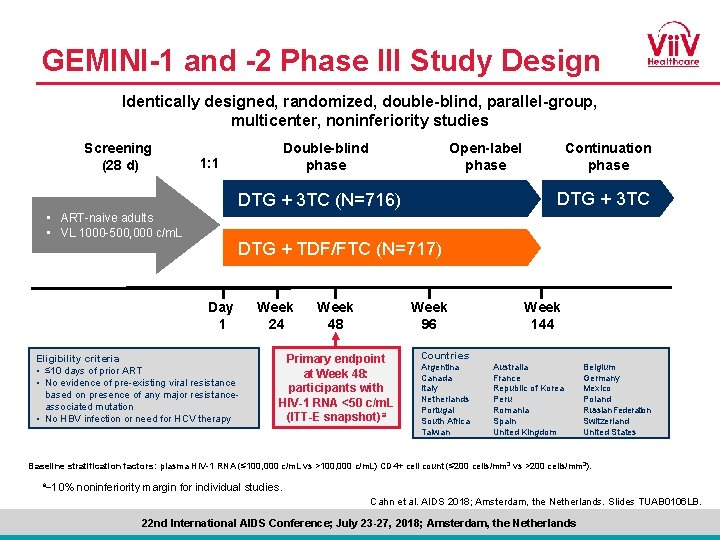
GEMINI-1 and -2 Phase III Study Design Identically designed, randomized, double-blind, parallel-group, multicenter, noninferiority studies Screening (28 d) Double-blind phase 1: 1 Open-label phase DTG + 3 TC (N=716) • ART-naive adults • VL 1000 -500, 000 c/m. L Continuation phase DTG + TDF/FTC (N=717) Day 1 Eligibility criteria • ≤ 10 days of prior ART • No evidence of pre-existing viral resistance based on presence of any major resistanceassociated mutation • No HBV infection or need for HCV therapy Week 24 Week 48 Week 96 Primary endpoint at Week 48: participants with HIV-1 RNA <50 c/m. L (ITT-E snapshot)a Week 144 Countries Argentina Canada Italy Netherlands Portugal South Africa Taiwan Australia France Republic of Korea Peru Romania Spain United Kingdom Belgium Germany Mexico Poland Russian Federation Switzerland United States Baseline stratification factors: plasma HIV-1 RNA (≤ 100, 000 c/m. L vs >100, 000 c/m. L) CD 4+ cell count (≤ 200 cells/mm 3 vs >200 cells/mm 3). a− 10% noninferiority margin for individual studies. Cahn et al. AIDS 2018; Amsterdam, the Netherlands. Slides TUAB 0106 LB. 22 nd International AIDS Conference; July 23 -27, 2018; Amsterdam, the Netherlands

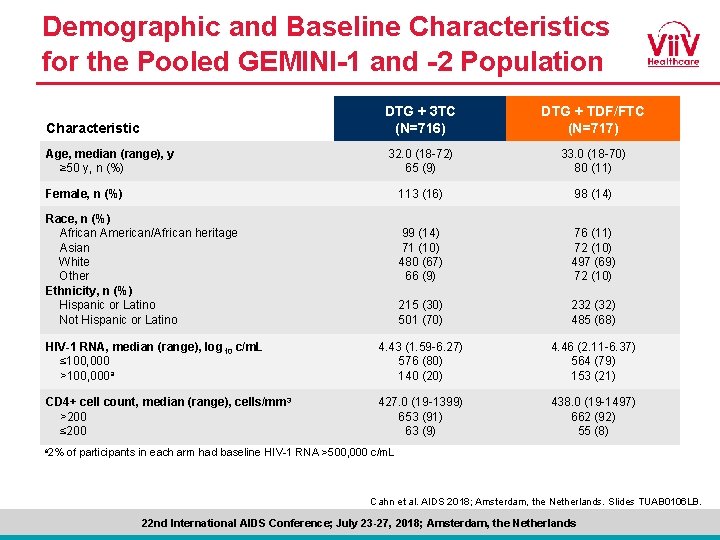
Demographic and Baseline Characteristics for the Pooled GEMINI-1 and -2 Population Characteristic DTG + 3 TC (N=716) DTG + TDF/FTC (N=717) Age, median (range), y ≥ 50 y, n (%) 32. 0 (18 -72) 65 (9) 33. 0 (18 -70) 80 (11) 113 (16) 98 (14) 99 (14) 71 (10) 480 (67) 66 (9) 76 (11) 72 (10) 497 (69) 72 (10) 215 (30) 501 (70) 232 (32) 485 (68) HIV-1 RNA, median (range), log 10 c/m. L ≤ 100, 000 >100, 000 a 4. 43 (1. 59 -6. 27) 576 (80) 140 (20) 4. 46 (2. 11 -6. 37) 564 (79) 153 (21) CD 4+ cell count, median (range), cells/mm 3 >200 ≤ 200 427. 0 (19 -1399) 653 (91) 63 (9) 438. 0 (19 -1497) 662 (92) 55 (8) Female, n (%) Race, n (%) African American/African heritage Asian White Other Ethnicity, n (%) Hispanic or Latino Not Hispanic or Latino a 2% of participants in each arm had baseline HIV-1 RNA >500, 000 c/m. L Cahn et al. AIDS 2018; Amsterdam, the Netherlands. Slides TUAB 0106 LB. 22 nd International AIDS Conference; July 23 -27, 2018; Amsterdam, the Netherlands

Snapshot Outcomes at Week 48 for GEMINI-1 Virologic outcome GEMINI-1 HIV-1 RNA <50 c/m. L, % 100 DTG + 3 TC (N=356) 90 Adjusted treatment difference (95% CI)a DTG + TDF/FTC (N=358) 93 + TDF/FTC DTGDTG + TDF/FTC GEMINI-1 -2. 6 -6. 7 80 DTG + 3 TC 1. 5 60 40 -10 -8 20 4 2 6 6 -6 -4 -2 0 2 4 6 Percentage-point difference 8 10 0 Virologic success Virologic nonresponse No virologic data a. Based on Cochran-Mantel-Haenszel stratified analysis adjusting for the following baseline stratification factors: plasma HIV-1 RNA (≤ 100, 000 c/m. L vs >100, 000 c/m. L) and CD 4+ cell count (≤ 200 cells/mm 3 vs >200 cells/mm 3). Cahn et al. AIDS 2018; Amsterdam, the Netherlands. Slides TUAB 0106 LB. 22 nd International AIDS Conference; July 23 -27, 2018; Amsterdam, the Netherlands

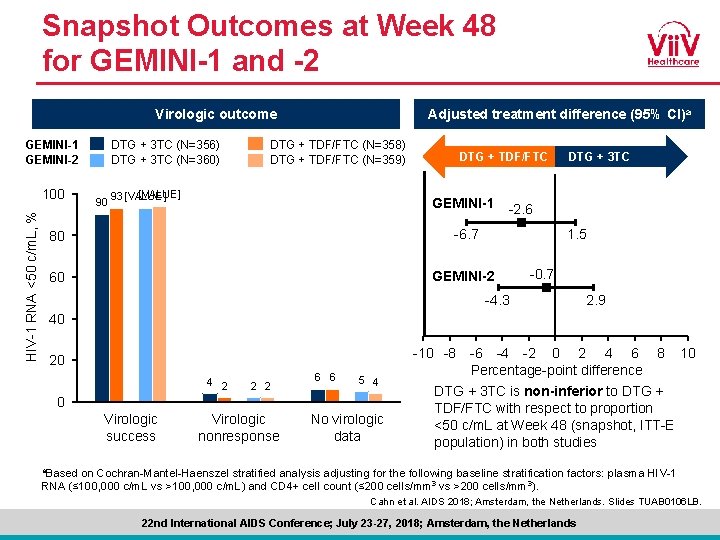
Snapshot Outcomes at Week 48 for GEMINI-1 and -2 Virologic outcome GEMINI-1 GEMINI-2 HIV-1 RNA <50 c/m. L, % 100 DTG + 3 TC (N=356) DTG + 3 TC (N=360) 90 Adjusted treatment difference (95% CI)a DTG + TDF/FTC (N=358) DTG + TDF/FTC (N=359) [VALUE] 93 [VALUE] + TDF/FTC DTGDTG + TDF/FTC GEMINI-1 80 -6. 7 60 GEMINI-2 DTG + 3 TC -2. 6 1. 5 -0. 7 -4. 3 2. 9 40 -10 -8 20 4 2 2 2 6 6 5 4 0 Virologic success Virologic nonresponse No virologic data -6 -4 -2 0 2 4 6 Percentage-point difference 8 10 DTG + 3 TC is non-inferior to DTG + TDF/FTC with respect to proportion <50 c/m. L at Week 48 (snapshot, ITT-E population) in both studies a. Based on Cochran-Mantel-Haenszel stratified analysis adjusting for the following baseline stratification factors: plasma HIV-1 RNA (≤ 100, 000 c/m. L vs >100, 000 c/m. L) and CD 4+ cell count (≤ 200 cells/mm 3 vs >200 cells/mm 3). Cahn et al. AIDS 2018; Amsterdam, the Netherlands. Slides TUAB 0106 LB. 22 nd International AIDS Conference; July 23 -27, 2018; Amsterdam, the Netherlands

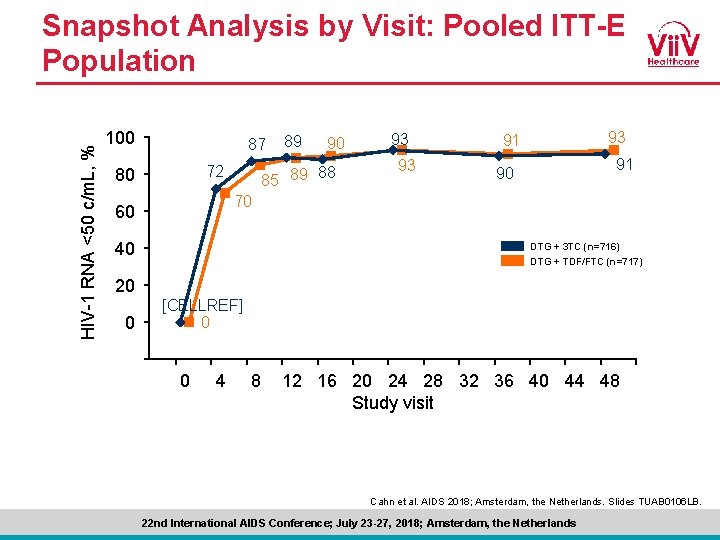
HIV-1 RNA <50 c/m. L, % Snapshot Analysis by Visit: Pooled ITT-E Population 100 87 72 80 89 90 85 89 88 93 93 93 91 91 90 70 60 40 DTG + 3 TC (n=716) DTG + TDF/FTC (n=717) 20 [CELLREF] 0 0 -20 -4 0 4 8 12 16 20 24 28 32 36 40 44 48 Study visit Cahn et al. AIDS 2018; Amsterdam, the Netherlands. Slides TUAB 0106 LB. 22 nd International AIDS Conference; July 23 -27, 2018; Amsterdam, the Netherlands

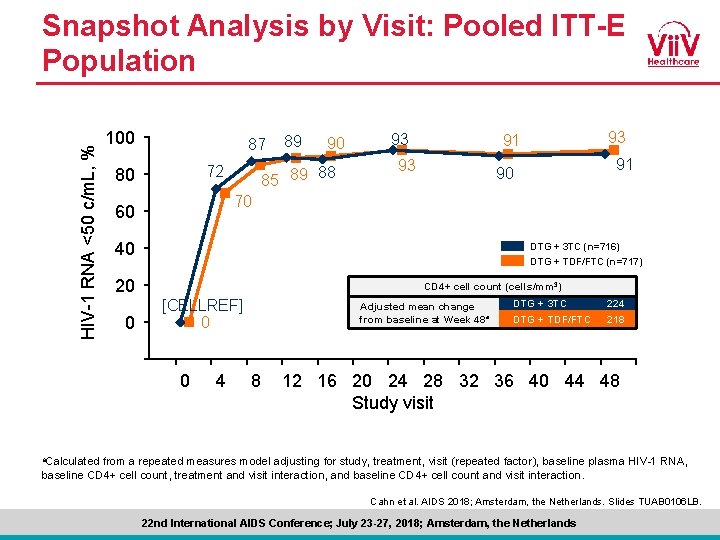
HIV-1 RNA <50 c/m. L, % Snapshot Analysis by Visit: Pooled ITT-E Population 100 87 72 80 89 90 85 89 88 93 93 91 90 70 60 40 DTG + 3 TC (n=716) DTG + TDF/FTC (n=717) 20 CD 4+ cell count (cells/mm 3) [CELLREF] 0 0 Adjusted mean change from baseline at Week 48 a DTG + 3 TC 224 DTG + TDF/FTC 218 -20 -4 0 4 8 12 16 20 24 28 32 36 40 44 48 Study visit a. Calculated from a repeated measures model adjusting for study, treatment, visit (repeated factor), baseline plasma HIV-1 RNA, baseline CD 4+ cell count, treatment and visit interaction, and baseline CD 4+ cell count and visit interaction. Cahn et al. AIDS 2018; Amsterdam, the Netherlands. Slides TUAB 0106 LB. 22 nd International AIDS Conference; July 23 -27, 2018; Amsterdam, the Netherlands

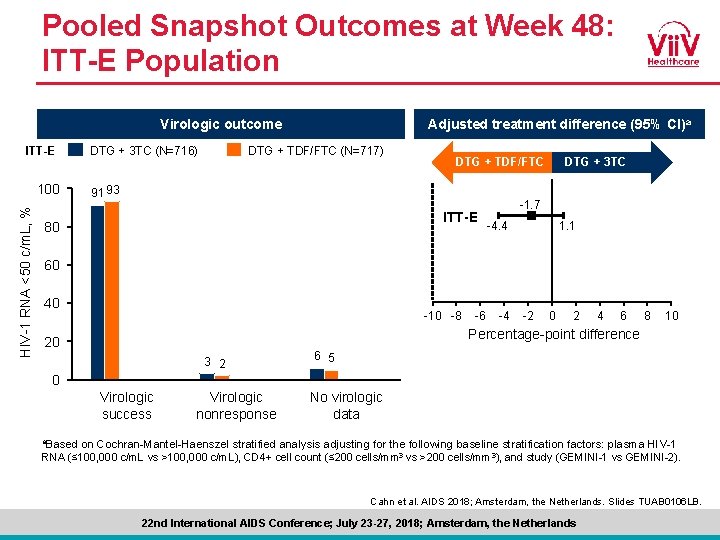
Pooled Snapshot Outcomes at Week 48: ITT-E Population Virologic outcome ITT-E HIV-1 RNA <50 c/m. L, % 100 DTG + 3 TC (N=716) Adjusted treatment difference (95% CI)a DTG + TDF/FTC (N=717) DTG + TDF/FTC 91 93 ITT-E 80 DTG + 3 TC -1. 7 -4. 4 1. 1 60 40 -10 -8 -6 -4 -2 0 2 4 6 8 10 Percentage-point difference 20 3 2 6 5 0 Virologic success Virologic nonresponse No virologic data a. Based on Cochran-Mantel-Haenszel stratified analysis adjusting for the following baseline stratification factors: plasma HIV-1 RNA (≤ 100, 000 c/m. L vs >100, 000 c/m. L), CD 4+ cell count (≤ 200 cells/mm 3 vs >200 cells/mm 3), and study (GEMINI-1 vs GEMINI-2). Cahn et al. AIDS 2018; Amsterdam, the Netherlands. Slides TUAB 0106 LB. 22 nd International AIDS Conference; July 23 -27, 2018; Amsterdam, the Netherlands

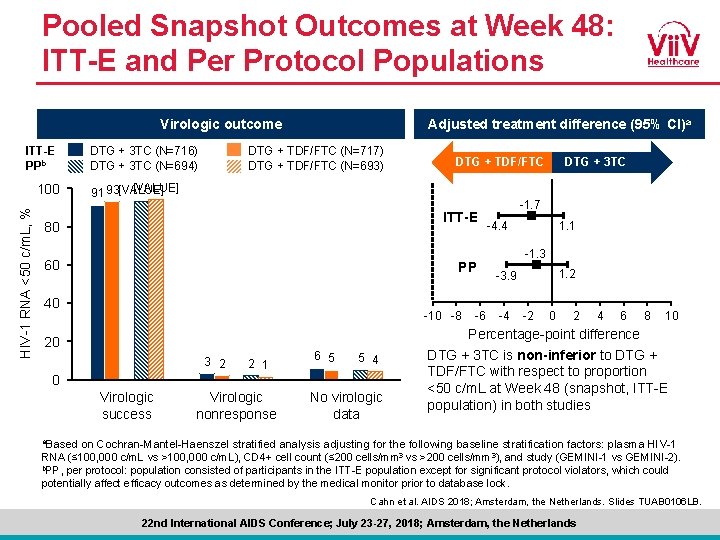
Pooled Snapshot Outcomes at Week 48: ITT-E and Per Protocol Populations Virologic outcome ITT-E PPb HIV-1 RNA <50 c/m. L, % 100 DTG + 3 TC (N=716) DTG + 3 TC (N=694) Adjusted treatment difference (95% CI)a DTG + TDF/FTC (N=717) DTG + TDF/FTC (N=693) DTG + TDF/FTC [VALUE] 91 93[VALUE] ITT-E 80 60 PP 40 -10 -8 20 3 2 2 1 6 5 5 4 0 Virologic success Virologic nonresponse No virologic data -6 DTG + 3 TC -1. 7 -4. 4 1. 1 -1. 3 1. 2 -3. 9 -4 -2 0 2 4 6 8 10 Percentage-point difference DTG + 3 TC is non-inferior to DTG + TDF/FTC with respect to proportion <50 c/m. L at Week 48 (snapshot, ITT-E population) in both studies a. Based on Cochran-Mantel-Haenszel stratified analysis adjusting for the following baseline stratification factors: plasma HIV-1 RNA (≤ 100, 000 c/m. L vs >100, 000 c/m. L), CD 4+ cell count (≤ 200 cells/mm 3 vs >200 cells/mm 3), and study (GEMINI-1 vs GEMINI-2). b. PP, per protocol: population consisted of participants in the ITT-E population except for significant protocol violators, which could potentially affect efficacy outcomes as determined by the medical monitor prior to database lock. Cahn et al. AIDS 2018; Amsterdam, the Netherlands. Slides TUAB 0106 LB. 22 nd International AIDS Conference; July 23 -27, 2018; Amsterdam, the Netherlands

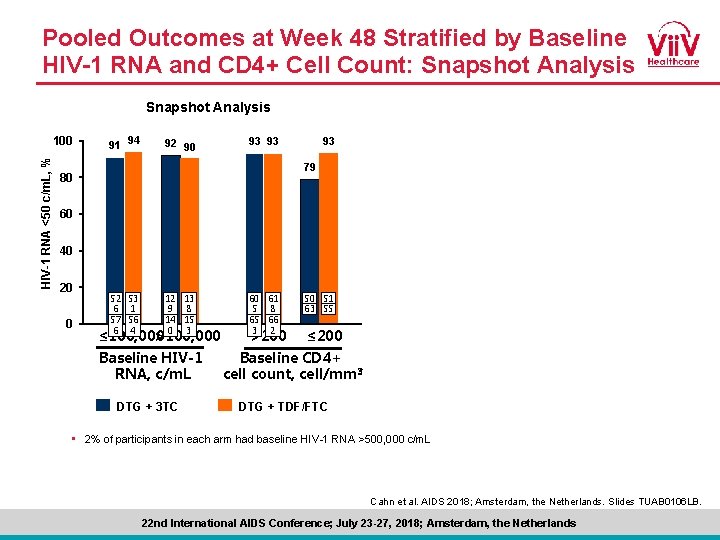
Pooled Outcomes at Week 48 Stratified by Baseline HIV-1 RNA: Snapshot Analysis HIV-1 RNA <50 c/m. L, % 100 91 94 92 90 93 93 93 79 80 60 40 20 0 52 53 6 1 57 56 6 4 12 13 9 8 14 15 0 3 ≤ 100, 000 >100, 000 Baseline HIV-1 RNA, c/m. L DTG + 3 TC 60 61 5 8 65 66 3 2 >200 50 51 67 55 ≤ 200 Baseline CD 4+ cell count, cell/mm 3 DTG + TDF/FTC • 2% of participants in each arm had baseline HIV-1 RNA >500, 000 c/m. L Cahn et al. AIDS 2018; Amsterdam, the Netherlands. Slides TUAB 0106 LB. 22 nd International AIDS Conference; July 23 -27, 2018; Amsterdam, the Netherlands

Pooled Outcomes at Week 48 Stratified by Baseline HIV-1 RNA and CD 4+ Cell Count: Snapshot Analysis HIV-1 RNA <50 c/m. L, % 100 91 94 92 90 93 93 93 79 80 60 40 20 0 52 53 6 1 57 56 6 4 12 13 9 8 14 15 0 3 ≤ 100, 000 >100, 000 Baseline HIV-1 RNA, c/m. L DTG + 3 TC 60 61 5 8 65 66 3 2 >200 50 51 63 55 ≤ 200 Baseline CD 4+ cell count, cell/mm 3 DTG + TDF/FTC • 2% of participants in each arm had baseline HIV-1 RNA >500, 000 c/m. L Cahn et al. AIDS 2018; Amsterdam, the Netherlands. Slides TUAB 0106 LB. 22 nd International AIDS Conference; July 23 -27, 2018; Amsterdam, the Netherlands

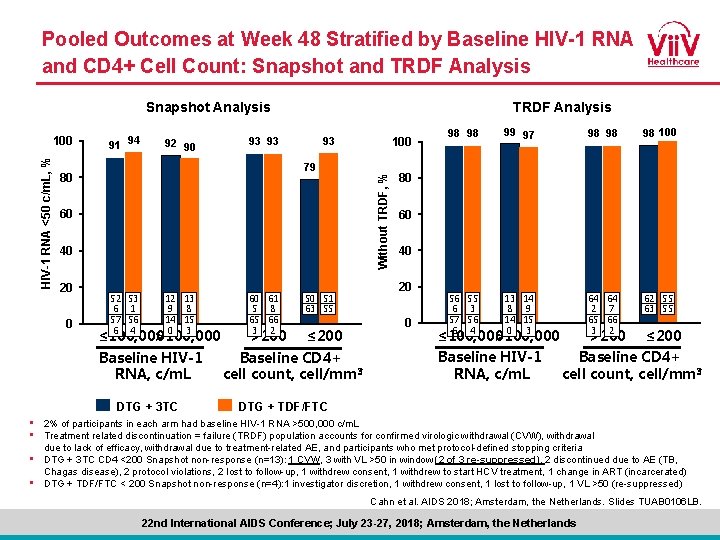
Pooled Outcomes at Week 48 Stratified by Baseline HIV-1 RNA and CD 4+ Cell Count: Snapshot and TRDF Analysis Snapshot Analysis 91 94 92 90 93 93 79 80 60 40 20 0 52 53 6 1 57 56 6 4 12 13 9 8 14 15 0 3 ≤ 100, 000 >100, 000 Baseline HIV-1 RNA, c/m. L DTG + 3 TC 60 61 5 8 65 66 3 2 >200 100 93 50 51 63 55 ≤ 200 Baseline CD 4+ cell count, cell/mm 3 Without TRDF, % HIV-1 RNA <50 c/m. L, % 100 TRDF Analysis 98 98 99 97 98 98 98 100 56 55 6 3 57 56 6 4 13 14 8 9 14 15 0 3 64 64 2 7 65 66 3 2 62 55 63 55 80 60 40 20 0 ≤ 100, 000 >100, 000 Baseline HIV-1 RNA, c/m. L >200 ≤ 200 Baseline CD 4+ cell count, cell/mm 3 DTG + TDF/FTC • 2% of participants in each arm had baseline HIV-1 RNA >500, 000 c/m. L • Treatment related discontinuation = failure (TRDF) population accounts for confirmed virologic withdrawal (CVW), withdrawal due to lack of efficacy, withdrawal due to treatment-related AE, and participants who met protocol-defined stopping criteria • DTG + 3 TC CD 4 <200 Snapshot non-response (n=13): 1 CVW, 3 with VL >50 in window (2 of 3 re-suppressed), 2 discontinued due to AE (TB, Chagas disease), 2 protocol violations, 2 lost to follow-up, 1 withdrew consent, 1 withdrew to start HCV treatment, 1 change in ART (incarcerated) • DTG + TDF/FTC < 200 Snapshot non-response (n=4): 1 investigator discretion, 1 withdrew consent, 1 lost to follow-up, 1 VL >50 (re-suppressed) Cahn et al. AIDS 2018; Amsterdam, the Netherlands. Slides TUAB 0106 LB. 22 nd International AIDS Conference; July 23 -27, 2018; Amsterdam, the Netherlands

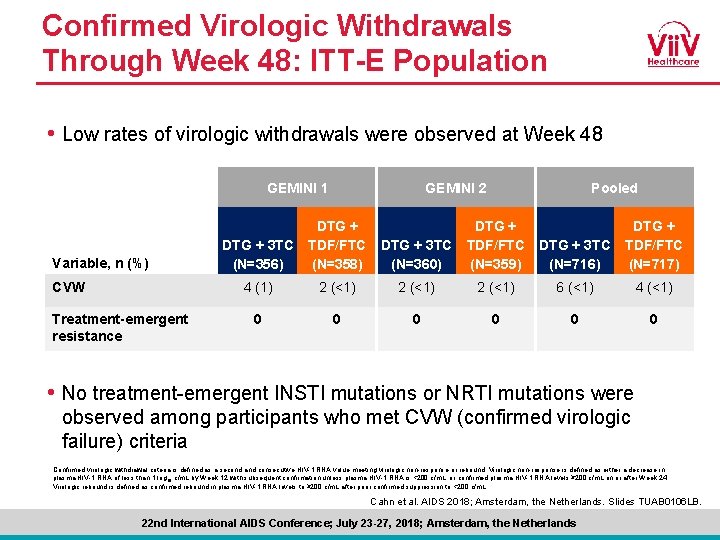
Confirmed Virologic Withdrawals Through Week 48: ITT-E Population • Low rates of virologic withdrawals were observed at Week 48 GEMINI 1 Variable, n (%) CVW Treatment-emergent resistance GEMINI 2 DTG + 3 TC (N=356) DTG + TDF/FTC (N=358) 4 (1) 0 Pooled DTG + 3 TC (N=360) DTG + TDF/FTC (N=359) DTG + 3 TC (N=716) DTG + TDF/FTC (N=717) 2 (<1) 6 (<1) 4 (<1) 0 0 0 • No treatment-emergent INSTI mutations or NRTI mutations were observed among participants who met CVW (confirmed virologic failure) criteria Confirmed virologic withdrawal criteria is defined as a second and consecutive HIV-1 RNA value meeting virologic non-response or rebound. Virologic non-response is defined as either a decrease in plasma HIV-1 RNA of less than 1 log 10 c/m. L by Week 12 with subsequent confirmation unless plasma HIV-1 RNA is <200 c/m. L, or confirmed plasma HIV-1 RNA levels ≥ 200 c/m. L on or after Week 24. Virologic rebound is defined as confirmed rebound in plasma HIV-1 RNA levels to ≥ 200 c/m. L after prior confirmed suppression to <200 c/m. L. . Cahn et al. AIDS 2018; Amsterdam, the Netherlands. Slides TUAB 0106 LB. 22 nd International AIDS Conference; July 23 -27, 2018; Amsterdam, the Netherlands

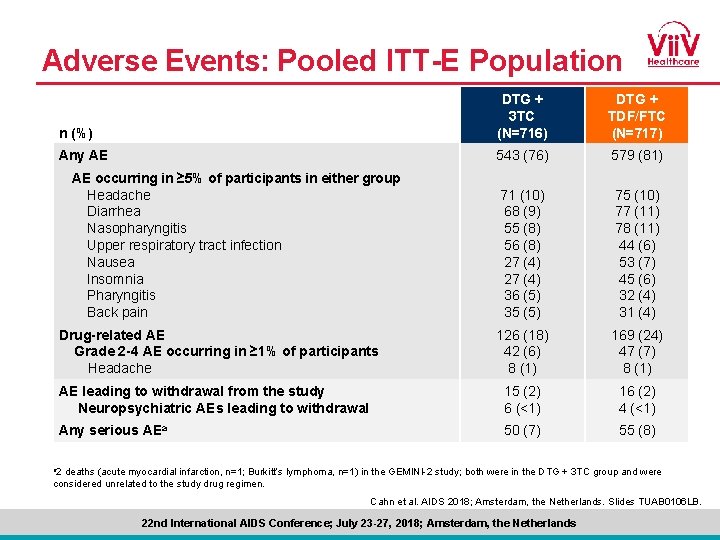
Adverse Events: Pooled ITT-E Population n (%) DTG + 3 TC (N=716) DTG + TDF/FTC (N=717) Any AE 543 (76) 579 (81) 71 (10) 68 (9) 55 (8) 56 (8) 27 (4) 36 (5) 35 (5) 75 (10) 77 (11) 78 (11) 44 (6) 53 (7) 45 (6) 32 (4) 31 (4) 126 (18) 42 (6) 8 (1) 169 (24) 47 (7) 8 (1) AE leading to withdrawal from the study Neuropsychiatric AEs leading to withdrawal 15 (2) 6 (<1) 16 (2) 4 (<1) Any serious AEa 50 (7) 55 (8) AE occurring in ≥ 5% of participants in either group Headache Diarrhea Nasopharyngitis Upper respiratory tract infection Nausea Insomnia Pharyngitis Back pain Drug-related AE Grade 2 -4 AE occurring in ≥ 1% of participants Headache a 2 deaths (acute myocardial infarction, n=1; Burkitt’s lymphoma, n=1) in the GEMINI-2 study; both were in the DTG + 3 TC group and were considered unrelated to the study drug regimen. Cahn et al. AIDS 2018; Amsterdam, the Netherlands. Slides TUAB 0106 LB. 22 nd International AIDS Conference; July 23 -27, 2018; Amsterdam, the Netherlands

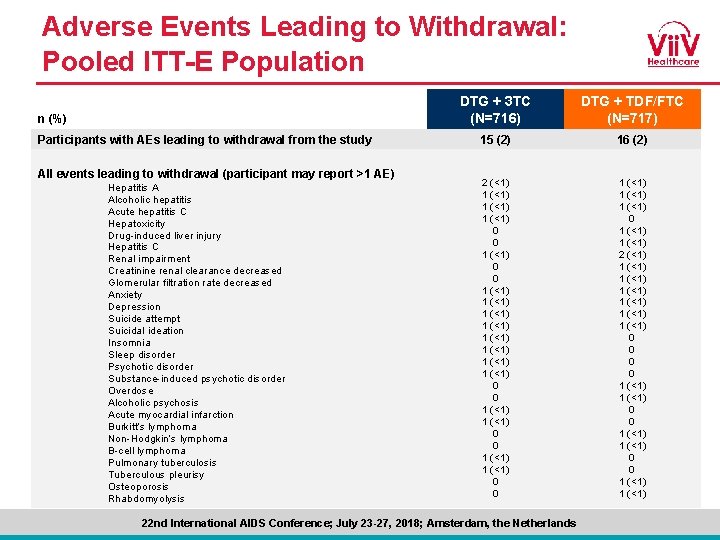
Adverse Events Leading to Withdrawal: Pooled ITT-E Population n (%) Participants with AEs leading to withdrawal from the study DTG + 3 TC (N=716) DTG + TDF/FTC (N=717) 15 (2) 16 (2) All events leading to withdrawal (participant may report >1 AE) Hepatitis A Alcoholic hepatitis Acute hepatitis C Hepatoxicity Drug-induced liver injury Hepatitis C Renal impairment Creatinine renal clearance decreased Glomerular filtration rate decreased Anxiety Depression Suicide attempt Suicidal ideation Insomnia Sleep disorder Psychotic disorder Substance-induced psychotic disorder Overdose Alcoholic psychosis Acute myocardial infarction Burkitt’s lymphoma Non-Hodgkin’s lymphoma B-cell lymphoma Pulmonary tuberculosis Tuberculous pleurisy Osteoporosis Rhabdomyolysis 2 (<1) 1 (<1) 1 (<1) 0 0 1 (<1) 2 (<1) 0 1 (<1) 1 (<1) 1 (<1) 0 1 (<1) 0 1 (<1) 0 1 (<1) 0 0 1 (<1) Cahn et al. AIDS 2018; Amsterdam, the Netherlands. Slides TUAB 0106 LB. 22 nd International AIDS Conference; July 23 -27, 2018; Amsterdam, the Netherlands

Change in Renal Biomarkers at Week 48: Pooled ITT-E Population Plasma/Serum markers Urine markers *p<0. 001 a 13. 5 15 10. 4 * 6. 3 4. 1 * 0 -5 -10 -12. 1 -15 31. 2 30 GFR from cystatin C, CKD-EPI (m. L/min/ 1. 73 m 2) Creatinine (µmol/L) GFR from creatinine, CKD-EPI (m. L/min/1. 73 m 2) DTG + 3 TC (n=716) * 20 11. 4 * 10 2. 9 0 -15. 5 -20 * 40 Change from baseline, %b Adjusted mean change from baselinea * -7. 4 -7. 7 -13. 1 -20 Protein/ Creatinine (g/mol) Retinol-binding protein/ Creatinine (µg/mmol) Beta-2 microglobulin/ Creatinine (mg/mmol) DTG + TDF/FTC (n=717) a. Estimated mean change from baseline at Week 48 in each arm calculated from ANCOVA model adjusting for: study, treatment, baseline plasma HIV-1 RNA, baseline CD 4+ cell count, age, sex, race, presence of diabetes mellitus, presence of hypertension, and baseline biomarker value. Multiple imputed dataset (missing at random). b. Estimated from geometric mean ratio for baseline and Week 48. Cahn et al. AIDS 2018; Amsterdam, the Netherlands. Slides TUAB 0106 LB. 22 nd International AIDS Conference; July 23 -27, 2018; Amsterdam, the Netherlands

Change in Bone Markers at Week 48: Pooled ITT-E Population Adjusted mean change from baseline (µg/L)a *p<0. 001 * 15 [VALUE] 10 * * 5 DTG + 3 TC (n=716) DTG + TDF/FTC (n=717) [VALUE] * [VALUE][VALUE] 0 Serum bone specific alkaline phosphatase Serum osteocalcin Serum procollagen 1 Serum type 1 collagen N-terminal propeptide C-telopeptide a. Estimated mean change from baseline at Week 48 in each arm calculated from ANCOVA model adjusting for study, treatment, baseline plasma HIV-1 RNA, baseline CD 4+ cell count, age, sex, race, BMI, smoking status, current vitamin D use, and baseline biomarker value. Multiple imputed dataset (missing at random). Cahn et al. AIDS 2018; Amsterdam, the Netherlands. Slides TUAB 0106 LB. 22 nd International AIDS Conference; July 23 -27, 2018; Amsterdam, the Netherlands

Conclusions • GEMINI-1 and-2 results demonstrate noninferior virologic efficacy for the 2 DR DTG + 3 TC vs the 3 DR DTG + TDF/FTC at Week 48 • Both DTG + 3 TC and DTG + TDF/FTC were associated with low rates of confirmed virologic withdrawals through Week 48 – No treatment-emergent INSTI or NRTI mutations were observed among participants who met CVW criteria • Overall safety and tolerability profile at Week 48 was comparable between the 2 regimens – Fewer drug-related AEs with DTG + 3 TC – Change in renal and bone biomarkers significantly favors DTG + 3 TC • These data support DTG + 3 TC as an effective option for the treatment of HIV-1 infection Cahn et al. AIDS 2018; Amsterdam, the Netherlands. Slides TUAB 0106 LB. 22 nd International AIDS Conference; July 23 -27, 2018; Amsterdam, the Netherlands

Acknowledgements • We thank everyone who has contributed to the success of these studies, including – Vii. V/GSK clinical study teams – The GEMINI-1 and GEMINI-2 clinical investigators and their staff Argentina Cassetti David Figueras Figueroa Losso Lopardo Lupo Porteiro Sánchez Australia Bloch Cooper Finlayson Koh Lewis Mc. Mahon Moore Roth Belgium De Wit Florence Goffard Demeester Lacor Vandercam Vandekerckh ove Canada Angel Baril Conway De Pokomandy Szabo Walmsley France Bouchaud Chidiac Delobel Girard Goujard Katlama Molina Pialoux Philibert Germany Bogner Esser Krznaric Lehmann Rockstroh Spinner Stellbrink Stephan Stoehr Italy Antinori Barchi Caramello Castelli Cattelan D’Arminio Di Biargo Di Perri Gori Maggiolo Mussini Penco Puoti Rizzardini Gulminetti Lazzarin Quirino Sighinolfi Viale Mexico Amaya Tapia Andrade Villanueva Granados Reyes Sierra-Madero Perez Rios Santoscoy Gomez Netherlands Den Hollander Rijnders Peru Hidalgo Hercilla Illescas Poland Olczak Portugal Mansinho Pacheco Teófilo Saraiva da Cunha Sarmento e Castro Serrão Romania Arbune Jianu Preotescu Prisacariu Russia Belonosova Borodkina Chernova Gankina Kizhlo Kulagin Kurina Nagimova Pokrovsky Riamova Voronin Yakovlev South Africa Kaplan South Korea Lee Kim Kim Spain Antela Lopez Arribas Lopez Casado Osorio Castaño Carracedo De Los Santos Gil Estrada perez Falco Force Galinda Puerto Garcia Deltoro Gatell Goenaga Sanchez Knobel Lopez Bernaldo de Quiros Losa Garcia Masia Montero-Alsonso Ocampo Hermida Pasquau Portilla Sogorb Pulido Rivera Roman Santos Fernandez Torres Perea Troya Viciana Switzerland Calmy Hauser Fehr Taiwan Cheng Ko Lin Lu Hung Tseng Wang Wong Yang USA Arduino Benson Berhe Bredeek Benson Brinson Campbell Crofoot Cunningham De. Jesus Dretler USA (cont) Crofoot Cunningham De. Jesus Dretler Eron Fife Fichtenbaum Flamm Goldstein Hagins Hoffman-Terry Jayaweera Kinder Klein Mc. Donald Mills Nahass Ortiz Osiyemi Overton Parks Prelutsky Ramgopal Schrader USA (cont) Simon Sims Skiest Slim Tashima Thedinger United Kingdom Clarke Gazzard Fox Johnson Kegg Khoo Mazhude Orkin Schembri Ustianowski Special recognition to the people living with HIV who have generously participated in the GEMINI trials 22 nd International AIDS Conference; July 23 -27, 2018; Amsterdam, the Netherlands

BACKUP SLIDES 22 nd International AIDS Conference; July 23 -27, 2018; Amsterdam, the Netherlands

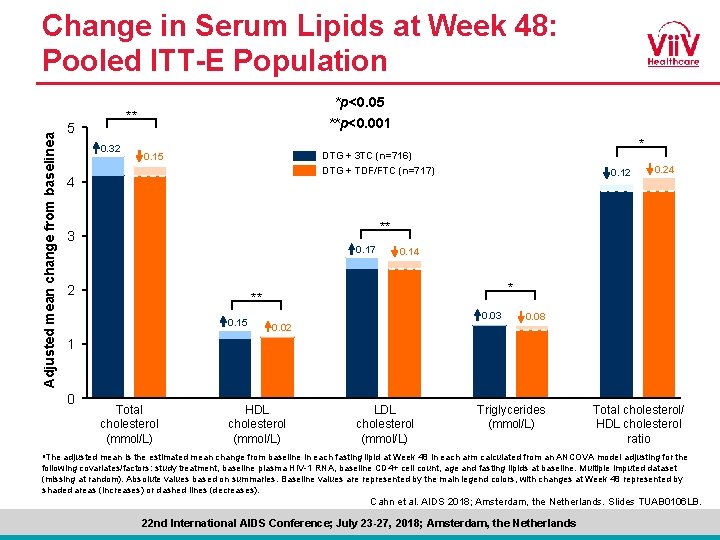
Adjusted mean change from baselinea Change in Serum Lipids at Week 48: Pooled ITT-E Population *p<0. 05 **p<0. 001 ** 5 0. 32 * DTG + 3 TC (n=716) DTG + TDF/FTC (n=717) 0. 15 4 0. 12 0. 24 ** 3 0. 17 2 0. 14 * ** 0. 15 0. 03 0. 02 0. 08 1 0 Total cholesterol (mmol/L) HDL cholesterol (mmol/L) LDL cholesterol (mmol/L) Triglycerides (mmol/L) Total cholesterol/ HDL cholesterol ratio a. The adjusted mean is the estimated mean change from baseline in each fasting lipid at Week 48 in each arm calculated from an ANCOVA model adjusting for the following covariates/factors: study treatment, baseline plasma HIV-1 RNA, baseline CD 4+ cell count, age and fasting lipids at baseline. Multiple imputed dataset (missing at random). Absolute values based on summaries. Baseline values are represented by the main legend colors, with changes at Week 48 represented by shaded areas (increases) or dashed lines (decreases). Cahn et al. AIDS 2018; Amsterdam, the Netherlands. Slides TUAB 0106 LB. 22 nd International AIDS Conference; July 23 -27, 2018; Amsterdam, the Netherlands
 Dolutegravir lamivudine
Dolutegravir lamivudine Lamivudine renal dose
Lamivudine renal dose Dtg plus
Dtg plus Dolutegravir + lamivudina + tenofovir
Dolutegravir + lamivudina + tenofovir Dtg test
Dtg test Tdf ftc dtg
Tdf ftc dtg Dtg/rpv
Dtg/rpv Dtg/rpv
Dtg/rpv Dtg/rpv
Dtg/rpv Dtg 3
Dtg 3 Dtg transport
Dtg transport Curva dtg
Curva dtg Tc gemini
Tc gemini Lms
Lms Dtg edf
Dtg edf Self-efficacy theory
Self-efficacy theory Albert bandura self efficacy
Albert bandura self efficacy Potency vs efficacy
Potency vs efficacy Efficacy potency
Efficacy potency Luminous efficacy comparison chart
Luminous efficacy comparison chart Collective teacher efficacy
Collective teacher efficacy Self efficacy definition
Self efficacy definition Vaccine efficacy
Vaccine efficacy