The Mathematics of Chemistry Significant Figures Uncertainty in


















- Slides: 23

The Mathematics of Chemistry Significant Figures

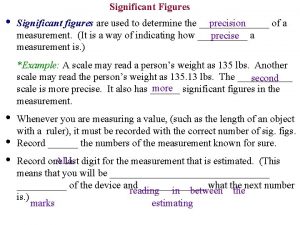
Uncertainty in Measurement • Measurements always have uncertainty. • Significant figures are the number of digits that are certain (can be measured) and the first uncertain digit.

Accuracy and Precision • Accuracy refers to how closely a measurement agrees with the accepted or true value. • Precision refers to reproducibility of measurements. • Chemistry calculations utilize significant figures to communicate uncertainty.

Rules for Significant Figures: 1. Non-zero digits and zeros between non-zero digits are always significant. 2. Leading zeros are not significant. 3. Zeros to the right of all non-zero digits are only significant if a decimal point is shown.

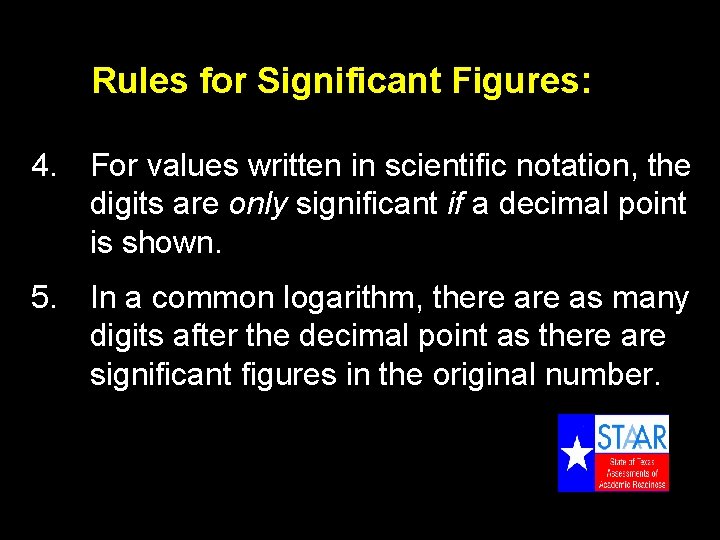
Rules for Significant Figures: 4. For values written in scientific notation, the digits are only significant if a decimal point is shown. 5. In a common logarithm, there as many digits after the decimal point as there are significant figures in the original number.

Rules for Finding Significant Figures Rule #1 - Non-zero digits and zeros between non-zero digits are always significant. 00340. 003210

Rules for Finding Significant Figures Rule #1 - Non-zero digits and zeros between non-zero digits are always significant. 00340. 003210

Rules for Finding Significant Figures Rule #2 - Zeros to the right of all nonzero digits are only significant if a decimal point is shown. 00340. 003210

Rules for Finding Significant Figures These zeros are not significant. There is not a rule that supports counting them. 00340. 003210

How many significant figures? 00340. 0 4 Rule #3

How many significant figures? 800. 1 4 Rule #1

How many significant figures? 0800. 10 5 Rules # 1 and 3

How many significant figures? 800 1 Rule #3

How many significant figures? 800. 3 Rule #3

How many significant figures? 0. 008 1 Rule #2

How many significant figures? 0. 180 3 Rule # 3

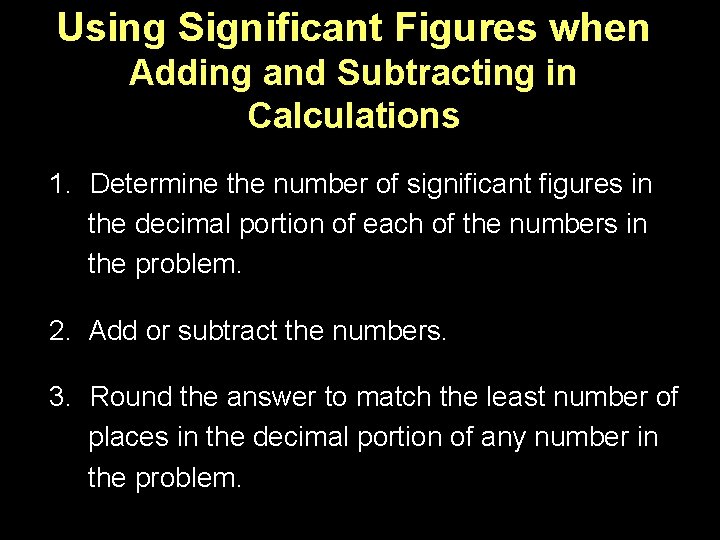
Using Significant Figures when Adding and Subtracting in Calculations 1. Determine the number of significant figures in the decimal portion of each of the numbers in the problem. 2. Add or subtract the numbers. 3. Round the answer to match the least number of places in the decimal portion of any number in the problem.

Using Significant Figures when Adding and Subtracting Give it a try! Add 0. 03 g of Na. Cl to 155 g of water. What is the total mass? Answer: 155 g because the mass of water has no decimal places, so the final answer must be written with no decimal places.

Using Significant Figures when Adding and Subtracting 892. 542 g 20. 629 g 0. 18 g + 4. 20 g 3 3 2 2 917. 551 The least amount of significant figures to the right of the decimal in the numbers is 2; therefore, the answer should only have 2 significant figures to the right of the decimal. 917. 55 g

Using Significant Figures when Multiplying and Dividing • Determine how many significant figures each numbers being multiplied or divided has, and note which number has the fewest. • Complete the calculation. • Write the answer using the same number of significant figures as the least number of significant figures found in the numbers used in the calculation.

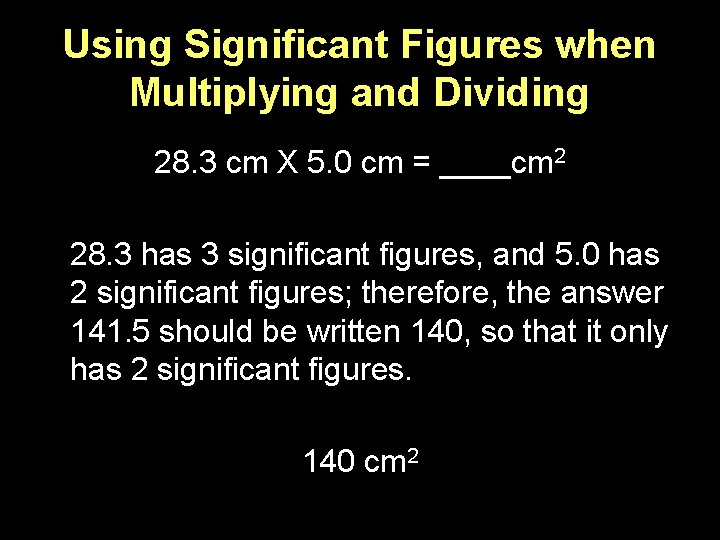
Using Significant Figures when Multiplying and Dividing 28. 3 cm X 5. 0 cm = ____cm 2 28. 3 has 3 significant figures, and 5. 0 has 2 significant figures; therefore, the answer 141. 5 should be written 140, so that it only has 2 significant figures. 140 cm 2

Try it! 454. 02 g of aluminum hydroxide multiplied by 5. 2 g equals how many grams? 454. 02 g X 5. 2 g = _____ g Rule: Write the answer using the same number of significant figures as the least number of significant figures found in the numbers used in the calculation.

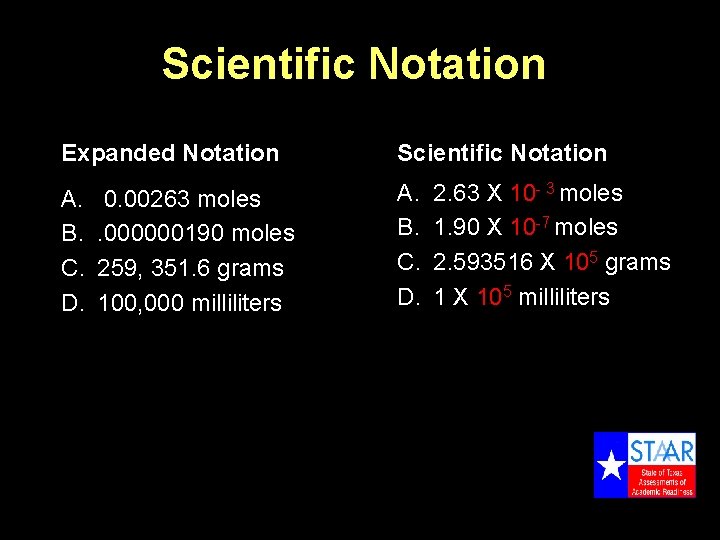
Scientific Notation Expanded Notation Scientific Notation A. B. C. D. 0. 00263 moles. 000000190 moles 259, 351. 6 grams 100, 000 milliliters 2. 63 X 10 - 3 moles 1. 90 X 10 -7 moles 2. 593516 X 105 grams 1 X 105 milliliters
 Uncertainty significant figures
Uncertainty significant figures What is a significant figure in chemistry
What is a significant figure in chemistry Significant figures ap chemistry
Significant figures ap chemistry 4 significant figures
4 significant figures Whats a sigfig
Whats a sigfig Sigfig rules
Sigfig rules What is a significant figure in chemistry
What is a significant figure in chemistry Rules for significant figures
Rules for significant figures Uncertainty in measurement and significant digits
Uncertainty in measurement and significant digits When is zero a significant figure
When is zero a significant figure Significant figures of 546 km
Significant figures of 546 km How many significant figures are there in 4 702 000 cm?
How many significant figures are there in 4 702 000 cm? Scientific notation significant figures worksheet
Scientific notation significant figures worksheet Non zero digits
Non zero digits Why significant figures are important
Why significant figures are important Significant figures characteristics
Significant figures characteristics Logarithm significant figures
Logarithm significant figures Significant figures of whole numbers
Significant figures of whole numbers Significant figures rules
Significant figures rules Significant figures for ph
Significant figures for ph Rounding off rules for 5
Rounding off rules for 5 Reflection about significant figures
Reflection about significant figures Significant figures review
Significant figures review 654 to 1 significant figure
654 to 1 significant figure